Cancer Res Treat.
2005 Feb;37(1):54-62.
cDNA Microarray Analysis of Differential Gene Expression in Gastric Cancer Cells Sensitive and Resistant to 5-Fluorouracil and Cisplatin
- Affiliations
-
- 1Department of Internal Medicine, Hanyang University College of Medicine, Korea. ahnmj@hanyang.ac.kr
- 2Department of Biochemistry, Hanyang University College of Medicine, Korea.
- 3Korea University Cancer Institute, College of Medicine, Korea University, Seoul, Korea.
Abstract
- PURPOSE
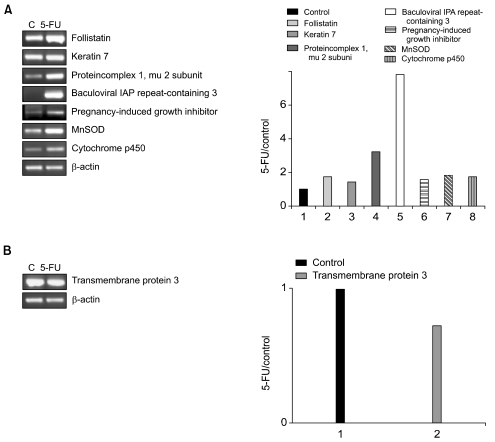
Gastric cancer is one of the most prevalent cancers worldwide. 5-fluorouracil (5-FU) and cisplatin are the most commonly used drugs for the treatment of gastric cancer. However, a significant number of tumors often fail to respond to chemotherapy. MATERIALS AND METHODS: To better understand the molecular mechanisms underlying drug resistance in gastric cancer the gene expression in gastric cancer cells, which were either sensitive or resistant to 5-FU and cisplatin, were examined using cDNA microarray analysis. To confirm the differential gene expression, as determined using the microarray, semiquantitative RT-PCR was performed on a subset of differentially expressed cDNAs. RESULTS: 69 and 45 genes, which were either up-regulated (9 and 22 genes) or down-regulated (60 and 25 genes), were identified in 5-FU- and cisplatin-resistant cells, respectively. Several genes, such as adaptor-related protein complex 1 and baculoviral IAP repeat-containing 3, were up-regulated in both drug-resistant cell types. Several genes, such as the ras homolog gene family, tropomyosin, tumor rejection antigen, protein disulfide isomerase-related protein, melanocortin 1 receptor, defensin, cyclophilin B, dual specificity phosphatase 8 and hepatocyte nuclear factor 3, were down-regulated in both drug-resistant cell types. CONCLUSION: These findings show that cDNA microarray analysis can be used to obtain gene expression profiles that reflect the effect of anticancer drugs on gastric cancer cells. Such data may lead to the assigning of signature expression profiles of drug-resistant tumors, which may help predict responses to drugs and assist in the design of tailored therapeutic regimens to overcome drug resistance.
MeSH Terms
-
Adaptor Protein Complex 1
Cisplatin*
Cyclophilins
DNA, Complementary*
Drug Resistance
Drug Therapy
Dual-Specificity Phosphatases
Fluorouracil*
Gene Expression*
Hepatocytes
Humans
Oligonucleotide Array Sequence Analysis*
Receptor, Melanocortin, Type 1
Stomach Neoplasms*
Transcriptome
Tropomyosin
Adaptor Protein Complex 1
Cisplatin
Cyclophilins
DNA, Complementary
Dual-Specificity Phosphatases
Fluorouracil
Receptor, Melanocortin, Type 1
Tropomyosin
Figure
Reference
-
1. Neugut AI, Hayek M, Howe G. Epidemiology of gastric cancer. Semin Oncol. 1996; 23:281–291. PMID: 8658212.2. Gottesman MM, Pastan I, Ambudkar SV. P-glycoprotein and mutidrug resistance. Curr Opin Genet Dev. 1996; 6:610–617. PMID: 8939727.3. Loe DW, Deeley RG, Cole SP. Biology of the multidrug resistance-associated protein, MRP. Eur J Cancer. 1991; 32A:945–957. PMID: 8763335.
Article4. Chaney SG, Sancar A. DNA repair: enzymatic mechanisms and relevance to drug response. J Natl Cancer Inst. 1996; 88:1346–1360. PMID: 8827012.
Article5. DeRisi J, Penland L, Brown PO, Bittner ML, Meltzer PS, Ray M, et al. Use of a cDNA microarray to analyse gene expression patterns in human cancer. Nat Genet. 1996; 14:457–460. PMID: 8944026.
Article6. DeRisi JL, Iyer VR, Brown PO. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997; 278:680–686. PMID: 9381177.
Article7. Marton MJ, DeRisi JL, Bennett HA, Iyer VR, Meyer MR, Roberts CJ, et al. Drug target validation and identification of secondary drug target effects using DNA microarrays. Nat Med. 1998; 4:1293–1301. PMID: 9809554.
Article8. Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, et al. The transcriptional program in the response of human fibroblasts to serum. Science. 1999; 283:83–87. PMID: 9872747.
Article9. Ahn MJ, Lee KH, Ahn JI, Yu DH, Lee HS, Choi JH, et al. The differential gene expression profiles between sensitive and resistant breast cancer cells to adriamycin by c DNA microarray. Cancer Res Treat. 2004; 36:43–49.10. Chung YM, Park S, Park JK, Kim Y, Kang Y, Yoo YD. Establishment and characterization of 5-fluorouracil-resistant gastric cancer cells. Cancer Lett. 2000; 159:95–101. PMID: 10974411.
Article11. Lee KH, Chang MY, Ahn JI, Yu DH, Jung SS, Chio JH, et al. Differential gene expression in retinoic acid-induced differenti ation of acute promyelocyteic leukemia cells, NB4 and HL-60 cells. Biochem Biophys Res Commun. 2002; 296:1125–1133. PMID: 12207890.12. Verhagen AM, Coulson EJ, Vaux DL. Inhibitor of apoptosis proteins and their relatives: IAPs and other BIRPs. Genome Biol. 2001; 2:REVIEWS3009. PMID: 11516343.13. Drane P, Bravard A, Bouvard V, May E. Reciprocal down-regulation of p53 and SOD2 gene expression: implication in p53 mediated apoptosis. Oncogene. 2001; 20:430–439. PMID: 11313974.14. Sakai H, Shimizu T, Hori K, Ikari A, Asano S, Takeguchi N. Molecular and pharmacological properties of inwardly rectifying K+ channels of human lung cancer cells. Eur J Pharmacol. 2002; 435:125–133. PMID: 11821018.
Article15. Stringer BK, Cooper AG, Shepard SB. Overexpression of the G-protein inwardly rectifying potassium channel 1 (GIRK1) in primary breast carcinomas correlates with axillary lymph node metastasis. Cancer Res. 2001; 61:582–588. PMID: 11212253.16. Crawford RM, Budas GR, Jovanovic S, Ranki HJ, Wilson TJ, Davies AM, et al. M-LDH serves as a sarcolemmal K(ATP) channel subunit essential for cell protection against ischemia. EMBO J. 2002; 21:3936–3948. PMID: 12145195.
Article17. McFadyen MC, McLeod HL, Jackson FC, Melvin WT, Doehmer J, Murray GI. Cytochrome p450 CYP1B1 protein expression: a novel mechanism of anticancer drug resistance. Biochem Pharmacol. 2001; 62:207–212. PMID: 11389879.18. Brockdorff BL, Skouv J, Reiter BE, Lykkesfeldt AE. Increased expression of cytochrome p450 1A1 and 1B1 genes in antiestrogen-resistant human breast cancer cell lines. Int J Cancer. 2000; 88:902–906. PMID: 11093812.
Article19. Los G, Muggia FM. Platinum resistance. Experimental and clinical status. Hematol Oncol Clin North Am. 1994; 8:411–429. PMID: 8040146.
Article20. Oldenburg J, Begg AC, van Vugt MJ, Ruevekamp M, Schornagel JH, Pinedo HM, et al. Characterization of resistance mechanisms to cis-diamminedichloroplatinum (II) in three sublines of the CC531 colon adenocarcinoma cell line in vitro. Cancer Res. 1994; 54:487–493. PMID: 8275486.21. Los G, Yang F, Samimi G, Manorek G, Guerorguieva IM, Howell S, et al. Using mRNA expression profiling to determine anticancer drug efficacy. Cytometry. 2002; 47:66–71. PMID: 11774355.
Article22. Kudoh K, Ramanna M, Ravatn R, Elkahloun AG, Bittner ML, Meltzer PS, et al. Monitoring the expression profiles of doxorubicin-induced and doxorubicin-resistant cancer cells by cDNA microarray. Cancer Res. 2000; 60:4161–4166. PMID: 10945624.23. Watts GS, Futscher BW, Isett R, Gleason-Guzman M, Kunkel MW, Salmon SE. cDNA microarray analysis of multidrug resistance: doxorubicin selection produces multiple defects in apoptosis signaling pathways. J Pharmacol Exp Ther. 2001; 299:434–441. PMID: 11602652.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterization of RhoA-mediated Chemoresistance in Gastric Cancer Cells
- Gene Expression Changes of Drug-resistant Saos-2 Cells Induced by Anticancer Drug
- Analysis of Gene Expression in Helicobacter pylori-associated Nodular Gastritis in Children Using Microarray
- Maspin Suppresses Survival of Lung Cancer Cells through Modulation of Akt Pathway
- The Differential Gene Expression Profiles between Sensitive and Resistant Breast Cancer Cells to Adriamycin by cDNA Microarray