J Vet Sci.
2014 Mar;15(1):27-33. 10.4142/jvs.2014.15.1.27.
Cell proliferation and neuroblast differentiation in the dentate gyrus of high-fat diet-fed mice are increased after rosiglitazone treatment
- Affiliations
-
- 1Department of Anatomy and Cell Biology, College of Veterinary Medicine, and Research Institute for Veterinary Science, Seoul National University, Seoul 151-742, Korea. ysyoon@snu.ac.kr
- 2Department of Biochemistry and Molecular Biology, Research Institute of Oral Sciences, College of Dentistry, Gangneung-Wonju National University, Gangneung 210-702, Korea.
- 3Department of Anatomy and Physiology, College of Pharmacy, Dankook University, Cheonan 330-714, Korea.
- 4Department of Anatomy, College of Veterinary Medicine, Kangwon National University, Chuncheon 200-701, Korea.
- 5Department of Neurobiology, School of Medicine, Kangwon National University, Chuncheon 200-701, Korea.
- KMID: 1737607
- DOI: http://doi.org/10.4142/jvs.2014.15.1.27
Abstract
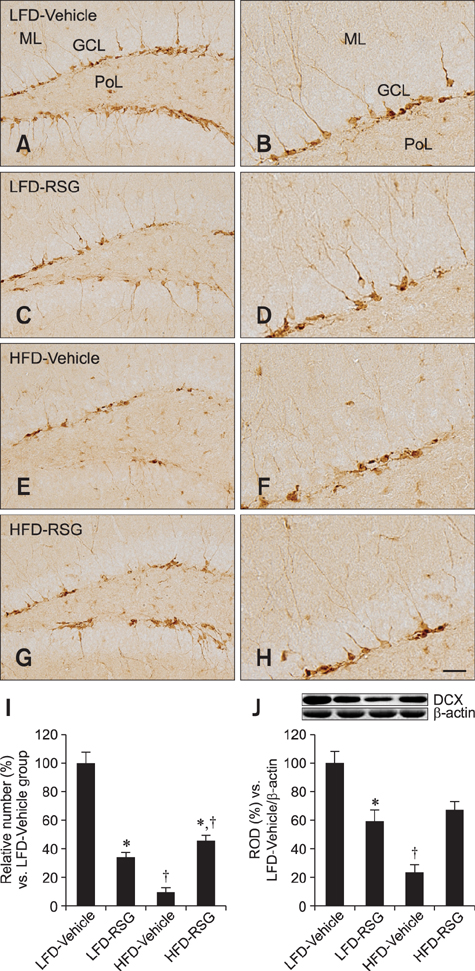
- In this study, we determined how rosiglitazone (RSG) differentially affected hippocampal neurogenesis in mice fed a low-fat diet (LFD) or high-fat diet (HFD; 60% fat). LFD and HFD were given to the mice for 8 weeks. Four weeks after initiating the LFD and HFD feeding, vehicle or RSG was administered orally once a day to both groups of mice. We measured cell proliferation and neuroblast differentiation in the subgranular zone of the dentate gyrus using Ki67 and doublecortin (DCX), respectively, as markers. In addition, we monitored the effects of RSG on the levels of DCX and brain-derived neurotrophic factor (BDNF) in hippocampal homogenates. At 8 weeks after the LFD feeding, the numbers of Ki67- and DCX-positive cells as well as hippocampal levels of DCX and BDNF were significantly decreased in the RSG-treated group compared to the vehicle-treated animals. In contrast, the numbers of Ki67- and DCX-positive cells along with hippocampal levels of DCX and BDNF in the HFD fed mice were significantly increased in the RSG-treated mice compared to the vehicle-treated group. Our data demonstrate that RSG can modulate the levels of BDNF, which could play a pivotal role in cell proliferation and neuroblast differentiation in the hippocampal dentate gyrus.
MeSH Terms
-
Animals
Blotting, Western
Brain-Derived Neurotrophic Factor/metabolism
Cell Differentiation/*drug effects
Cell Proliferation/drug effects
Dentate Gyrus/growth & development/physiology
Diet, Fat-Restricted
*Diet, High-Fat
Hippocampus/growth & development/physiology
Hypoglycemic Agents/*pharmacology
Immunohistochemistry
Ki-67 Antigen/metabolism
Male
Mice, Inbred C57BL
Microtubule-Associated Proteins/metabolism
Neurogenesis/*drug effects
Neuropeptides/metabolism
Thiazolidinediones/*pharmacology
Brain-Derived Neurotrophic Factor
Hypoglycemic Agents
Ki-67 Antigen
Microtubule-Associated Proteins
Neuropeptides
Thiazolidinediones
Figure
Reference
-
1. Boitard C, Etchamendy N, Sauvant J, Aubert A, Tronel S, Marighetto A, Layé S, Ferreira G. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus. 2012; 22:2095–2100.
Article2. Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003; 467:1–10.
Article3. Chan JP, Cordeira J, Calderon GA, Iyer LK, Rios M. Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol Cell Neurosci. 2008; 39:372–383.
Article4. Cooper-Kuhn CM, Kuhn HG. Is it all DNA repair? Methodological considerations for detecting neurogenesis in the adult brain. Brain Res Dev Brain Res. 2002; 134:13–21.5. Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004; 3:169–178.
Article6. Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998; 4:1313–1317.
Article7. Esposito G, Scuderi C, Valenza M, Togna GI, Latina V, De Filippis D, Cipriano M, Carratù MR, Iuvone T, Steardo L. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS One. 2011; 6:e28668.
Article8. Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press;1997.9. Giaginis C, Tsourouflis G, Theocharis S. Peroxisome proliferator-activated receptor-γ (PPAR-γ) ligands: novel pharmacological agents in the treatment of ischemia reperfusion injury. Curr Mol Med. 2008; 8:562–579.
Article10. Greenwood CE, Winocur G. High-fat diets, insulin resistance and declining cognitive function. Neurobiol Aging. 2005; 26:Suppl 1. 42–45.
Article11. Heneka MT, Landreth GE. PPARs in the brain. Biochim Biophys Acta. 2007; 1771:1031–1045.
Article12. Hwang IK, Kim IY, Kim DW, Yoo KY, Kim YN, Yi SS, Won MH, Lee IS, Yoon YS, Seong JK. Strain-specific differences in cell proliferation and differentiation in the dentate gyrus of C57BL/6N and C3H/HeN mice fed a high fat diet. Brain Res. 2008; 1241:1–6.
Article13. Hwang IK, Kim IY, Kim YN, Yi SS, Park IS, Min BH, Doo HK, Ahn SY, Kim YS, Lee IS, Yoon YS, Seong JK. Comparative study on high fat diet-induced 4-hydroxy-2E-nonenal adducts in the hippocampal CA1 region of C57BL/6N and C3H/HeN mice. Neurochem Res. 2009; 34:964–972.
Article14. Islam O, Loo TX, Heese K. Brain-derived neurotrophic factor (BDNF) has proliferative effects on neural stem cells through the truncated TRK-B receptor, MAP kinase, AKT, and STAT-3 signaling pathways. Curr Neurovasc Res. 2009; 6:42–53.
Article15. Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993; 60:9–26.
Article16. Jin J, Albertz J, Guo Z, Peng Q, Rudow G, Troncoso JC, Ross CA, Duan W. Neuroprotective effects of PPAR-γ agonist rosiglitazone in N171-82Q mouse model of Huntington's disease. J Neurochem. 2013; 125:410–419.
Article17. Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, Shiota M, Kesterson RA, Kahn BB, Magnuson MA. Deletion of PPARγ in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci U S A. 2005; 102:6207–6212.
Article18. Kanakasabai S, Pestereva E, Chearwae W, Gupta SK, Ansari S, Bright JJ. PPARγ agonists promote oligodendrocyte differentiation of neural stem cells by modulating stemness and differentiation genes. PLoS One. 2012; 7:e50500.
Article19. Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008; 13:1813–1826.
Article20. Kaundal RK, Sharma SS. Peroxisome proliferator-activated receptor gamma agonists as neuroprotective agents. Drug News Perspect. 2010; 23:241–256.
Article21. Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997; 94:10409–10414.22. Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997; 386:493–495.
Article23. Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000; 405:421–424.
Article24. Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Nagai R, Tobe K, Kimura S, Kadowaki T. PPARγ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999; 4:597–609.
Article25. Launer LJ. Demonstrating the case that AD is a vascular disease: epidemiologic evidence. Ageing Res Rev. 2002; 1:61–77.
Article26. Lee CH, Choi JH, Yoo KY, Park OK, Moon JB, Sohn Y, Cho JH, Hwang IK, Won MH. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor γ, decreases immunoreactivity of markers for cell proliferation and neuronal differentiation in the mouse hippocampus. Brain Res. 2010; 1329:30–35.
Article27. Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002; 82:1367–1375.
Article28. McTigue DM, Tripathi R, Wei P, Lash AT. The PPAR gamma agonist Pioglitazone improves anatomical and locomotor recovery after rodent spinal cord injury. Exp Neurol. 2007; 205:396–406.
Article29. Miglio G, Rattazzi L, Rosa AC, Fantozzi R. PPARγ stimulation promotes neurite outgrowth in SH-SY5Y human neuroblastoma cells. Neurosci Lett. 2009; 454:134–138.
Article30. Park HR, Park M, Choi J, Park KY, Chung HY, Lee J. A high-fat diet impairs neurogenesis: involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci Lett. 2010; 482:235–239.
Article31. Sairanen M, Lucas G, Ernfors P, Castrén M, Castrén E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005; 25:1089–1094.
Article32. Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, Carta AR. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson's disease. Eur J Neurosci. 2009; 29:954–963.
Article33. Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007; 18:391–418.
Article34. Sodhi RK, Singh N, Jaggi AS. Neuroprotective mechanisms of peroxisome proliferator-activated receptor agonists in Alzheimer's disease. Naunyn Schmiedebergs Arch Pharmacol. 2011; 384:115–124.
Article35. Sundararajan S, Landreth GE. Antiinflammatory properties of PPARgamma agonists following ischemia. Drug News Perspect. 2004; 17:229–236.
Article36. Vidal-Puig A, Jimenez-Liñan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE. Regulation of PPAR γ gene expression by nutrition and obesity in rodents. J Clin Invest. 1996; 97:2553–2561.
Article37. Wang S, Sun Z, Guo Y, Yuan Y, Li L. PPARγ-mediated advanced glycation end products regulation of neural stem cells. Mol Cell Endocrinol. 2009; 307:176–184.
Article38. Yoo DY, Kim W, Nam SM, Yoo KY, Lee CH, Choi JH, Won MH, Hwang IK, Yoon YS. Reduced cell proliferation and neuroblast differentiation in the dentate gyrus of high fat diet-fed mice are ameliorated by metformin and glimepiride treatment. Neurochem Res. 2011; 36:2401–2408.
Article39. Yoo DY, Shin BN, Kim IH, Kim W, Kim DW, Yoo KY, Choi JH, Lee CH, Yoon YS, Choi SY, Won MH, Hwang IK. Effects of Cu,Zn-superoxide dismutase on cell proliferation and neuroblast differentiation in the mouse dentate gyrus. Neurochem Res. 2012; 37:261–267.
Article40. Zhao X, Ou Z, Grotta JC, Waxham N, Aronowski J. Peroxisome-proliferator-activated receptor-gamma (PPARγ) activation protects neurons from NMDA excitotoxicity. Brain Res. 2006; 1073-1074:460–469.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vanillin and 4-hydroxybenzyl alcohol attenuate cognitive impairment and the reduction of cell proliferation and neuroblast differentiation in the dentate gyrus in a mouse model of scopolamine-induced amnesia
- Bacopa monnieri extract improves novel object recognition, cell proliferation, neuroblast differentiation, brain-derived neurotrophic factor, and phosphorylation of cAMP response element-binding protein in the dentate gyrus
- The high dosage of earthworm (Eisenia andrei) extract decreases cell proliferation and neuroblast differentiation in the mouse hippocampal dentate gyrus
- Temporal Change of Calbindin-D28k Immunoreactivity in the Dentate Gyrus of Voluntary Running Mouse
- Comparison of pharmacological and genetic inhibition of cyclooxygenase-2: effects on adult neurogenesis in the hippocampal dentate gyrus