Anat Cell Biol.
2011 Sep;44(3):218-225. 10.5115/acb.2011.44.3.218.
The high dosage of earthworm (Eisenia andrei) extract decreases cell proliferation and neuroblast differentiation in the mouse hippocampal dentate gyrus
- Affiliations
-
- 1Department of Neurobiology, Kangwon National University School of Medicine, Chuncheon, Korea. mhwon@kangwon.ac.kr
- 2Department of Oral Anatomy, College of Dentistry, Gangneung-Wonju National University, Gangneung, Korea.
- 3Laboratory of Veterinary Pharmacology, College of Veterinary Medicine, Seoul National University, Seoul, Korea.
- 4Department of Anatomy, College of Veterinary Medicine, Kangwon National University, Chuncheon, Korea.
- KMID: 1447434
- DOI: http://doi.org/10.5115/acb.2011.44.3.218
Abstract
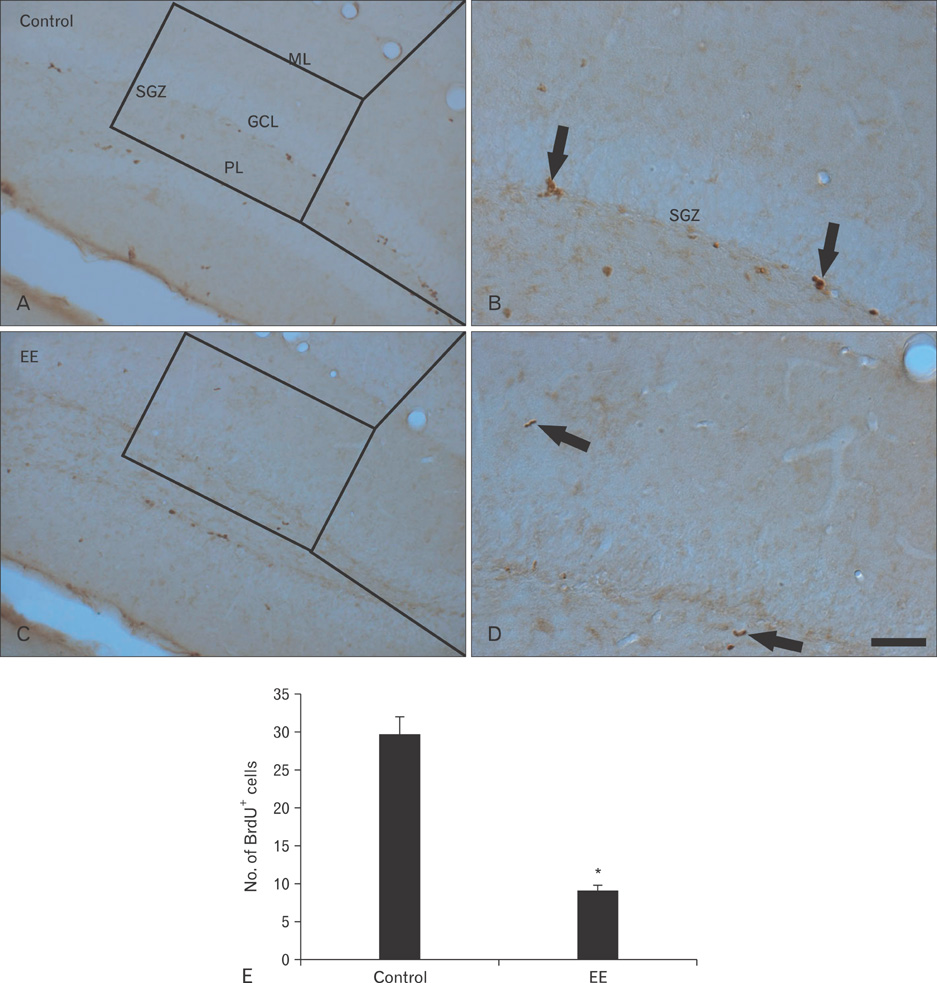
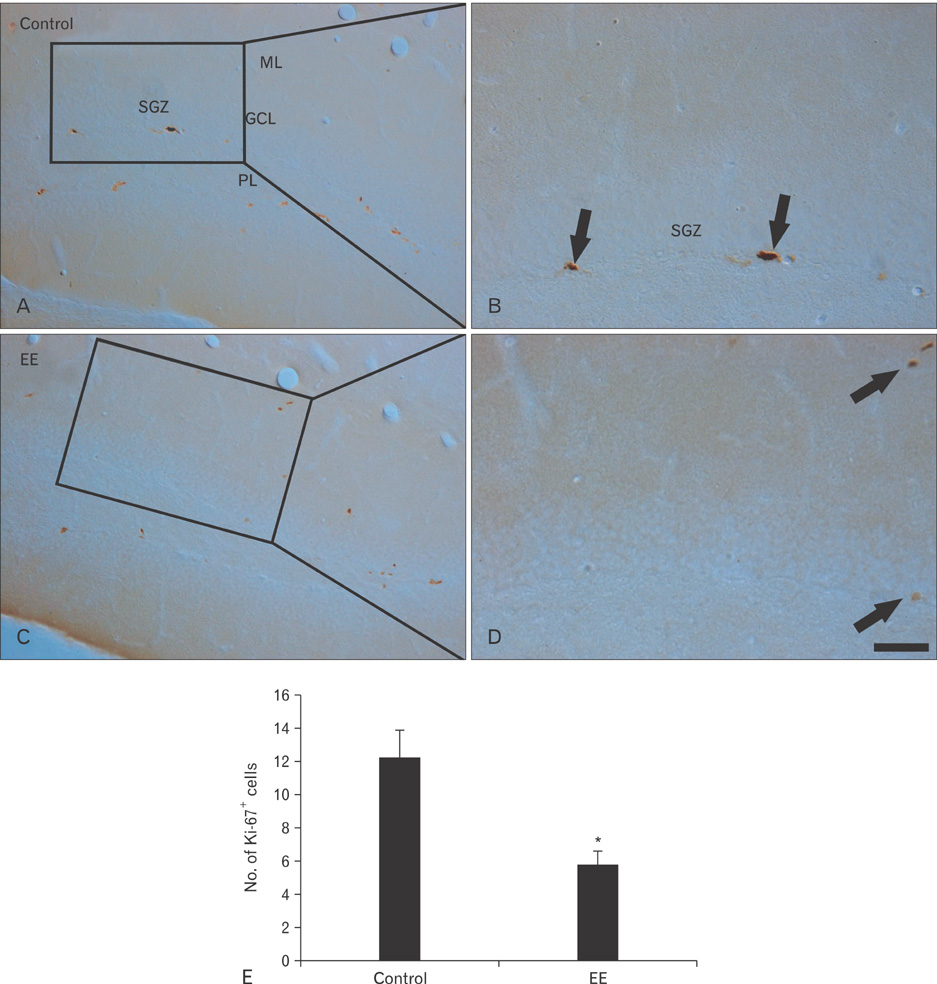
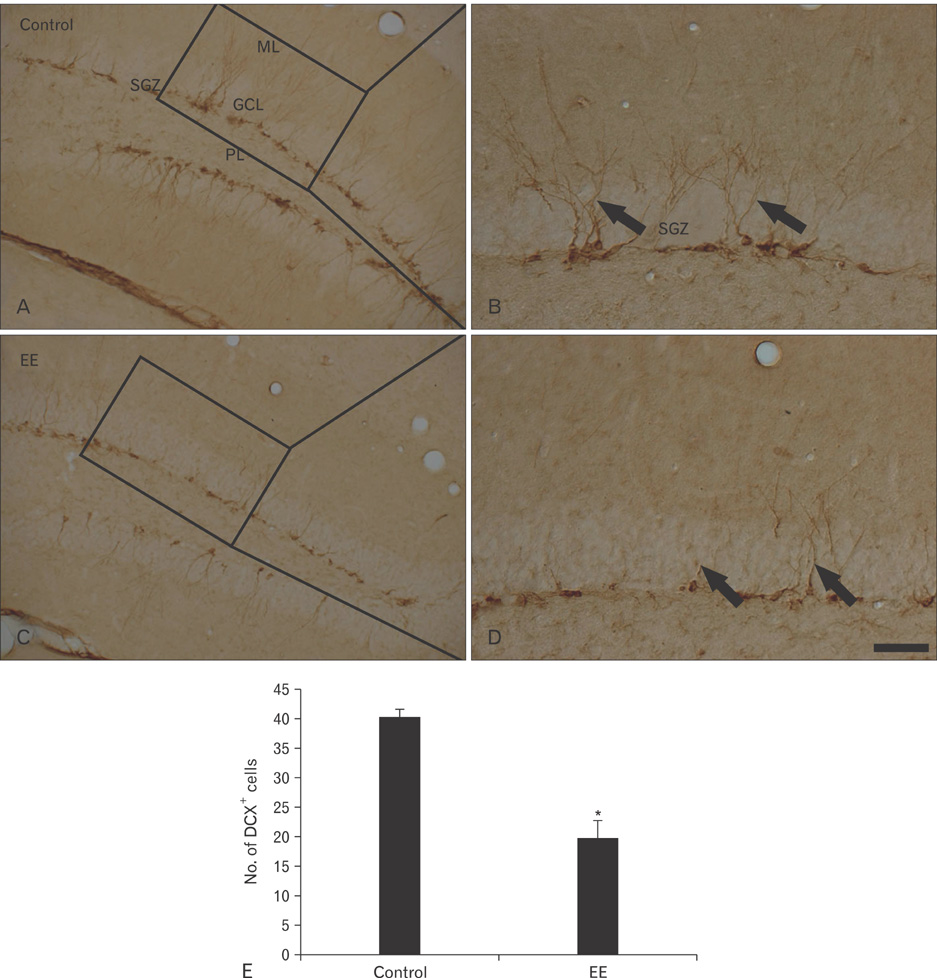
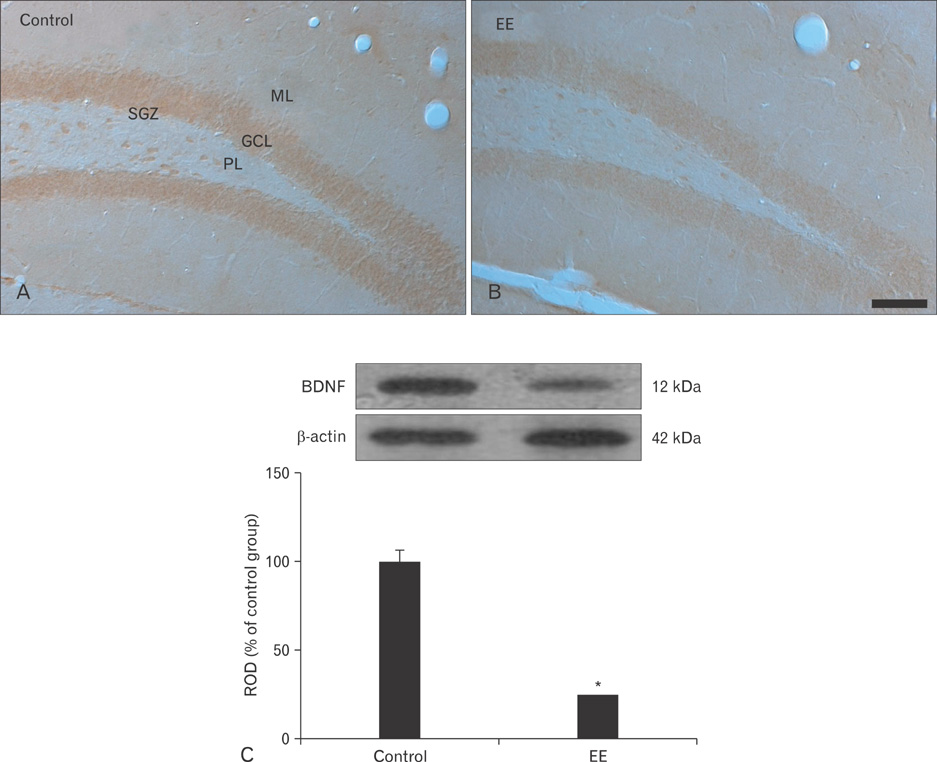
- Earthworm extract has shown anticancer characteristics. In the present study, we examined the effect of chronic treatment with a high dose of earthworm (Eisenia andrei) extract (EE) on cell proliferation and neuroblast differentiation in the hippocampal dentate gyrus (DG) of 3-week-old mice using 5-bromo-2'-deoxyuridine (BrdU) and Ki-67 immunohistochemistry for cell proliferation and doublecortin (DCX) immunohistochemistry for neuroblast differentiation, respectively. BrdU-, Ki-67-, and DCX-immunoreactive cells were easily detected in the subgranular zone of the DG in vehicle (saline)-treated mice. However, BrdU-, Ki-67-, and DCX-immunoreactive cells in the 500 mg/kg EE-treated mice decreased distinctively compared to those in the vehicle-treated mice. In addition, brain-derived neurotrophic factor (BDNF) immunoreactivity and its protein level decreased markedly in the DG of the EE-treated group compared to those in the vehicle-treated group. These results indicate that chronic treatment with high dose EE decreased cell proliferation and neuroblast differentiation, and that BDNF immunoreactivity decreased in the DG of EE-treated mice.
MeSH Terms
Figure
Reference
-
1. Ago Y, Yoneyama M, Ishihama T, Kataoka S, Kawada K, Tanaka T, Ogita K, Shintani N, Hashimoto H, Baba A, Takuma K, Matsuda T. Role of endogenous pituitary adenylate cyclase-activating polypeptide in adult hippocampal neurogenesis. Neuroscience. 2011. 172:554–561.2. Zou L, Jin G, Zhang X, Qin J, Zhu H, Tian M, Tan X. Proliferation, migration, and neuronal differentiation of the endogenous neural progenitors in hippocampus after fimbria fornix transection. Int J Neurosci. 2010. 120:192–200.3. Jaako-Movits K, Zharkovsky T, Pedersen M, Zharkovsky A. Decreased hippocampal neurogenesis following olfactory bulbectomy is reversed by repeated citalopram administration. Cell Mol Neurobiol. 2006. 26:1559–1570.4. Ma DK, Marchetto MC, Guo JU, Ming GL, Gage FH, Song H. Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat Neurosci. 2010. 13:1338–1344.5. Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer's disease: allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res. 2006. 3:185–190.6. Hong XP, Peng CX, Wei W, Tian Q, Liu YH, Cao FY, Wang Q, Wang JZ. Relationship of adult neurogenesis with tau phosphorylation and GSK-3β activity in subventricular zone. Neurochem Res. 2011. 36:288–296.7. Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006. 11:514–522.8. Yao RQ, Zhang L, Wang W, Li L. Cornel iridoid glycoside promotes neurogenesis and angiogenesis and improves neurological function after focal cerebral ischemia in rats. Brain Res Bull. 2009. 79:69–76.9. Balamurugan M, Parthasarathi K, Ranganathan LS, Cooper EL. Hypothetical mode of action of earthworm extract with hepatoprotective and antioxidant properties. J Zhejiang Univ Sci B. 2008. 9:141–147.10. Cooper EL. CAM, eCAM, bioprospecting: the 21st century pyramid. Evid Based Complement Alternat Med. 2005. 2:125–127.11. Popović M, Hrcenjak TM, Babić T, Kos J, Grdisa M. Effect of earthworm (G-90) extract on formation and lysis of clots originated from venous blood of dogs with cardiopathies and with malignant tumors. Pathol Oncol Res. 2001. 7:197–202.12. Ryu GH, Park S, Han DK, Kim YH, Min B. Antithrombotic activity of a lumbrokinase immobilized polyurethane surface. ASAIO J. 1993. 39:M314–M318.13. Cooper EL, Ru B, Weng N. Earthworms: sources of antimicrobial and anticancer molecules. Adv Exp Med Biol. 2004. 546:359–389.14. Kalluri HS, Dempsey RJ. D609 inhibits the proliferation of neural progenitor cells. Neuroreport. 2010. 21:700–703.15. Kim MS, Park HR, Park M, Kim SJ, Kwon M, Yu BP, Chung HY, Kim HS, Kwack SJ, Kang TS, Kim SH, Lee J. Neurotoxic effect of 2,5-hexanedione on neural progenitor cells and hippocampal neurogenesis. Toxicology. 2009. 260:97–103.16. Paizanis E, Kelaï S, Renoir T, Hamon M, Lanfumey L. Life-long hippocampal neurogenesis: environmental, pharmacological and neurochemical modulations. Neurochem Res. 2007. 32:1762–1771.17. Balamurugan M, Parthasarathi K, Cooper EL, Ranganathan LS. Anti-inflammatory and anti-pyretic activities of earthworm extract-Lampito mauritii (Kinberg). J Ethnopharmacol. 2009. 121:330–332.18. Hrzenjak T, Popović M, Bozić T, Grdisa M, Kobrehel D, Tiska-Rudman L. Fibrinolytic and anticoagulative activities from the earthworm Eisenia foetida. Comp Biochem Physiol B Biochem Mol Biol. 1998. 119:825–832.19. Zhang M, Li X, Liu Y, Ye F, Qiu G. Effects of extract of dilong (pheretima) on the scalded skin in rats. J Tradit Chin Med. 2006. 26:68–71.20. Lee CH, Choi JH, Yoo KY, Park OK, Moon JB, Sohn Y, Cho JH, Hwang IK, Won MH. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, decreases immunoreactivity of markers for cell proliferation and neuronal differentiation in the mouse hippocampus. Brain Res. 2010. 1329:30–35.21. Chen Y, Ai Y, Slevin JR, Maley BE, Gash DM. Progenitor proliferation in the adult hippocampus and substantia nigra induced by glial cell line-derived neurotrophic factor. Exp Neurol. 2005. 196:87–95.22. Sairanen M, Lucas G, Ernfors P, Castrén M, Castrén E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005. 25:1089–1094.23. Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001. 21:6706–6717.24. Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005. 192:348–356.25. Islam O, Loo TX, Heese K. Brain-derived neurotrophic factor (BDNF) has proliferative effects on neural stem cells through the truncated TRK-B receptor, MAP kinase, AKT, and STAT-3 signaling pathways. Curr Neurovasc Res. 2009. 6:42–53.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vanillin and 4-hydroxybenzyl alcohol attenuate cognitive impairment and the reduction of cell proliferation and neuroblast differentiation in the dentate gyrus in a mouse model of scopolamine-induced amnesia
- Bacopa monnieri extract improves novel object recognition, cell proliferation, neuroblast differentiation, brain-derived neurotrophic factor, and phosphorylation of cAMP response element-binding protein in the dentate gyrus
- Temporal Change of Calbindin-D28k Immunoreactivity in the Dentate Gyrus of Voluntary Running Mouse
- Comparison of pharmacological and genetic inhibition of cyclooxygenase-2: effects on adult neurogenesis in the hippocampal dentate gyrus
- Systemic administration of low dosage of tetanus toxin decreases cell proliferation and neuroblast differentiation in the mouse hippocampal dentate gyrus