Nutr Res Pract.
2022 Aug;16(4):435-449. 10.4162/nrp.2022.16.4.435.
Comparison of laxative effects of fermented soybeans (Cheonggukjang) containing toxins and biogenic amines against loperamide-induced constipation mouse model
- Affiliations
-
- 1Jeonju AgroBio-Materials Institute, Jeonju 54810, Korea
- 2Microbial Institute for Fermentation Industry, Sunchang 56048, Korea
- KMID: 2532094
- DOI: http://doi.org/10.4162/nrp.2022.16.4.435
Abstract
- BACKGROUND/OBJECTIVES
(Cheonggukjang) is a traditional fermented soybean paste with significant health-promoting effects. On the other hand, there have been insufficient studies on the safety and efficacy of (Cheonggukjang), which is produced using traditional methods containing toxins and biogenic amines (BAs). This study compared the laxative effect of (Cheonggukjang), containing high or low levels of toxins and BAs (HTBC or LTBC) in a loperamide (Lop)-induced constipation mouse model.
MATERIALS/METHODS
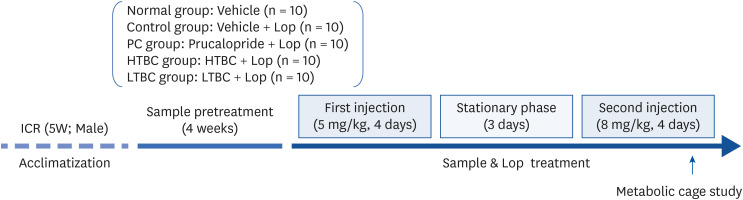
To induce constipation, Lop (5 mg/kg) was administered orally to ICR mice twice a day for 4 days, and the dose was increased to 8 mg/kg after a 3-day rest period. (Cheonggukjang) (500 mg/kg, HTBC, or LTBC respectively) was administered for four weeks before the Lop treatment.
RESULTS
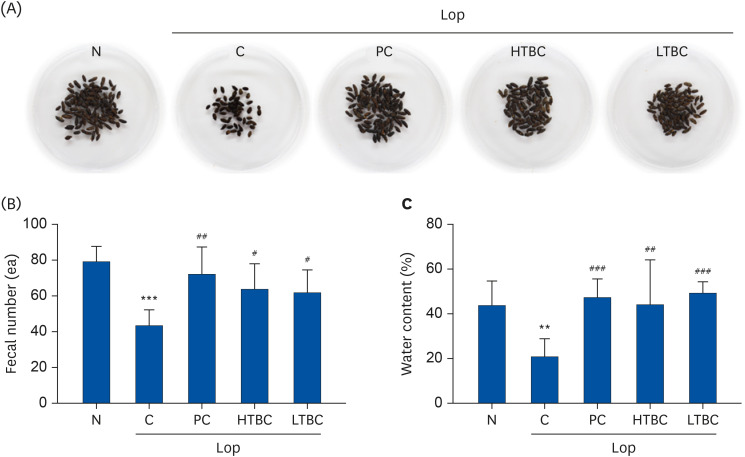
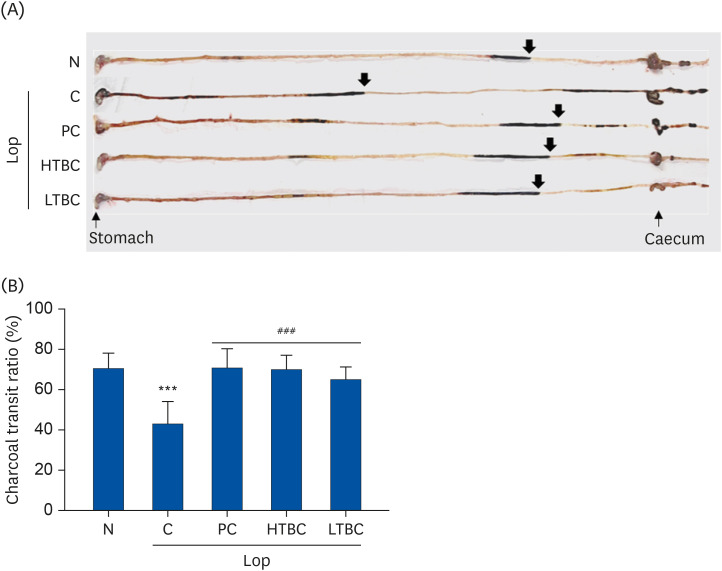
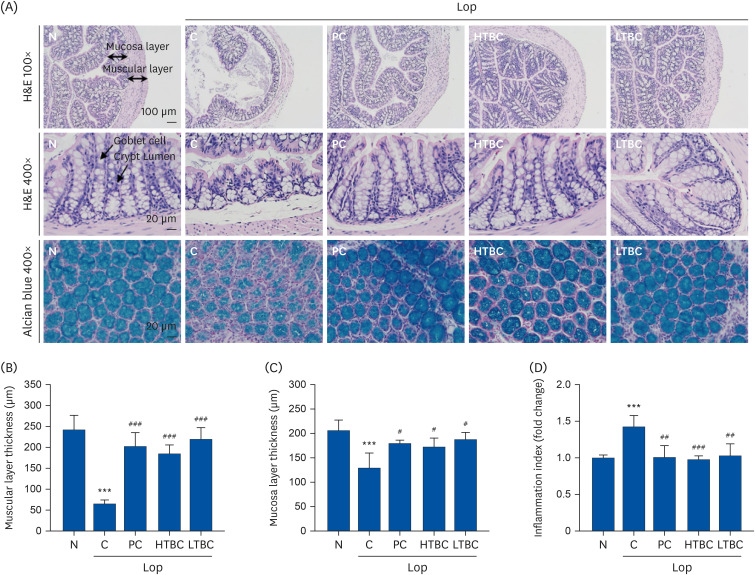
The number of stools, fecal weight, water contents, gastrointestinal transit, and histological alterations were recovered significantly in the HTBC or LTBC groups. HTBC and LTBC administration did not induce significant changes in body weight, dietary intake, and behavior. The opioid-receptor downstream signaling pathway in colon tissues was also evaluated. The c-Kit, stem cell kinase, and mitogen-activated protein kinases subfamilies, including extracellular signal-regulated kinase 1/2, c-Jun N-terminal kinases, and p38, were all downregulated in the HTBC or LTBC-administered mice colon compared to the Lop group.
CONCLUSION
These results show that (Cheonggukjang), containing high levels of toxins and BAs, have a similar laxative effect in a mouse model of Lop-induced constipation.
Keyword
Figure
Reference
-
1. Woo JK, Choi S, Kang JH, Kim DE, Hurh BS, Jeon JE, Kim SY, Oh SH. Fermented barley and soybean (BS) mixture enhances intestinal barrier function in dextran sulfate sodium (DSS)-induced colitis mouse model. BMC Complement Altern Med. 2016; 16:498. PMID: 27912750.
Article2. Chen L, Zhang J, Suo H, Wang W, Wang H, Zhang Y, Hu Q, Zhao X, Li J. Preventive effects of different fermentation times of Shuidouchi on diphenoxylate-induced constipation in mice. Foods. 2019; 8:86.
Article3. Park YK, Lee JH, Mah JH. Occurrence and reduction of biogenic amines in traditional Asian fermented soybean foods: a review. Food Chem. 2019; 278:1–9. PMID: 30583348.
Article4. ten Brink B, Damink C, Joosten HM, Huis in ’t Veld JH. Occurrence and formation of biologically active amines in foods. Int J Food Microbiol. 1990; 11:73–84. PMID: 2223522.
Article5. Mah JH, Park YK, Jin YH, Lee JH, Hwang HJ. Bacterial production and control of biogenic amines in Asian fermented soybean foods. Foods. 2019; 8:85.
Article6. Silla Santos MH. Biogenic amines: their importance in foods. Int J Food Microbiol. 1996; 29:213–231. PMID: 8796424.
Article7. Jeon AR, Lee JH, Mah JH. Biogenic amine formation and bacterial contribution in Cheonggukjang, a Korean traditional fermented soybean food. Lebensm Wiss Technol. 2018; 92:282–289.
Article8. Ibe A, Nishima T, Kasai N. Bacteriological properties of and amine-production conditions for tyramine-and histamine-producing bacterial strains isolated from soybean paste (miso) starting materials. Eisei kagaku. 1992; 38:403–409.
Article9. Lee YJ, Kim JE, Kwak MH, Go J, Son HJ, Kim DS, Kang BC, Lee HS, Hwang DY. Toxicity of fermented soybean product (Cheonggukjang) manufactured by mixed culture of Bacillus subtilis MC31 and Lactobacillus sakei 383 on liver and kidney of ICR mice. Lab Anim Res. 2014; 30:54–63. PMID: 24999359.
Article10. Shin D, Jeong D. Korean traditional fermented soybean products: Jang . J Ethn Foods. 2015; 2:2–7.11. Camilleri M, Ford AC, Mawe GM, Dinning PG, Rao SS, Chey WD, Simrén M, Lembo A, Young-Fadok TM, Chang L. Chronic constipation. Nat Rev Dis Primers. 2017; 3:17095. PMID: 29239347.
Article12. García-García P, Brenes-Balbuena M, Hornero-Méndez D, García-Borrego A, Garrido-Fernández A. Content of biogenic amines in table olives. J Food Prot. 2000; 63:111–116. PMID: 10643779.
Article13. Kim JE, Yun WB, Sung JE, Lee HA, Choi JY, Choi YS, Jung YS, Kim KS, Hwang DY. Characterization the response of Korl:ICR mice to loperamide induced constipation. Lab Anim Res. 2016; 32:231–240. PMID: 28053617.
Article14. Su H, Chen J, Miao S, Deng K, Liu J, Zeng S, Zheng B, Lu X. Lotus seed oligosaccharides at various dosages with prebiotic activity regulate gut microbiota and relieve constipation in mice. Food Chem Toxicol. 2019; 134:110838. PMID: 31568850.
Article15. Tan YY, Ji ZL, Zhao G, Jiang JR, Wang D, Wang JM. Decreased SCF/c-kit signaling pathway contributes to loss of interstitial cells of Cajal in gallstone disease. Int J Clin Exp Med. 2014; 7:4099–4106. PMID: 25550919.16. Ro S, Park C, Jin J, Zheng H, Blair PJ, Redelman D, Ward SM, Yan W, Sanders KM. A model to study the phenotypic changes of interstitial cells of Cajal in gastrointestinal diseases. Gastroenterology. 2010; 138:1068–1078.e1-2. PMID: 19917283.
Article17. Ihara E, Akiho H, Nakamura K, Turner SR, Macdonald JA. MAPKs represent novel therapeutic targets for gastrointestinal motility disorders. World J Gastrointest Pathophysiol. 2011; 2:19–25. PMID: 21607162.
Article18. Kwon DY, Chung KR, Jang DJ. The history and science of Chongkukjang, a Korean fermented soybean product. J Ethn Foods. 2019; 6:5.19. Park KY, Kim JY, Moon SH. Anti-obesity and anti- inflammatory effects of Cheonggukjang . FASEB J. 2011; 25:584.8.20. Choi JH, Pichiah PB, Kim MJ, Cha YS. Cheonggukjang, a soybean paste fermented with B. licheniformis-67 prevents weight gain and improves glycemic control in high fat diet induced obese mice. J Clin Biochem Nutr. 2016; 59:31–38. PMID: 27499576.
Article21. Soh J, Kwon DY, Cha YS. Hepatic gene expression profiles are altered by dietary unsalted Korean fermented soybean (Chongkukjang) consumption in mice with diet-induced obesity. J Nutr Metab. 2011; 2011:260214. PMID: 21437188.22. Oh SJ, Mah JH, Kim JH, Kim YW, Hwang HJ. Reduction of tyramine by addition of Schizandra chinensis baillon in Cheonggukjang . J Med Food. 2012; 15:1109–1115. PMID: 23216112.
Article23. Locke GR 3rd, Pemberton JH, Phillips SF. American Gastroenterological Association. AGA technical review on constipation. Gastroenterology. 2000; 119:1766–1778. PMID: 11113099.
Article24. Shimotoyodome A, Meguro S, Hase T, Tokimitsu I, Sakata T. Decreased colonic mucus in rats with loperamide-induced constipation. Comp Biochem Physiol A Mol Integr Physiol. 2000; 126:203–212. PMID: 10936760.
Article25. Wong BS, Manabe N, Camilleri M. Role of prucalopride, a serotonin (5-HT4) receptor agonist, for the treatment of chronic constipation. Clin Exp Gastroenterol. 2010; 3:49–56. PMID: 21694846.26. Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006; 68:307–343. PMID: 16460275.
Article27. Li F, Zhou H, Zhou X, Yi R, Mu J, Zhao X, Liu W. Lactobacillus plantarum CQPC05 isolated from pickled vegetables inhibits constipation in mice. Applied Sciences. 2019; 9:159.
Article28. Nelson AD, Camilleri M. Chronic opioid induced constipation in patients with nonmalignant pain: challenges and opportunities. Therap Adv Gastroenterol. 2015; 8:206–220.
Article29. Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001; 22:153–183. PMID: 11294822.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Corrosive Gastritis Caused by Salt-fermented Northern Sand Lance
- Effects of electroconvulsive shock on the levels of biogenic amines and their metabolits in rat brain
- Correlation between laxative effects of uridine and suppression of ER stress in loperamide induced constipated SD rats
- Laxative effect of peanut sprout extract
- Regulation of gastrointestinal hormones during laxative activity of gallotannin-enriched extract isolated from Galla Rhois in loperamide-induced constipation of SD rats