Korean Circ J.
2009 Jun;39(6):217-222. 10.4070/kcj.2009.39.6.217.
The Calcium and Voltage Clocks in Sinoatrial Node Automaticity
- Affiliations
-
- 1Krannert Institute of Cardiology and the Division of Cardiology, Department of Medicine, Indiana University School of Medicine, Indianapolis, USA. chenpp@iupui.edu
- 2Department of Cardiology, Fukuoka University School of Medicine, Fukuoka, Japan.
- KMID: 1490696
- DOI: http://doi.org/10.4070/kcj.2009.39.6.217
Abstract
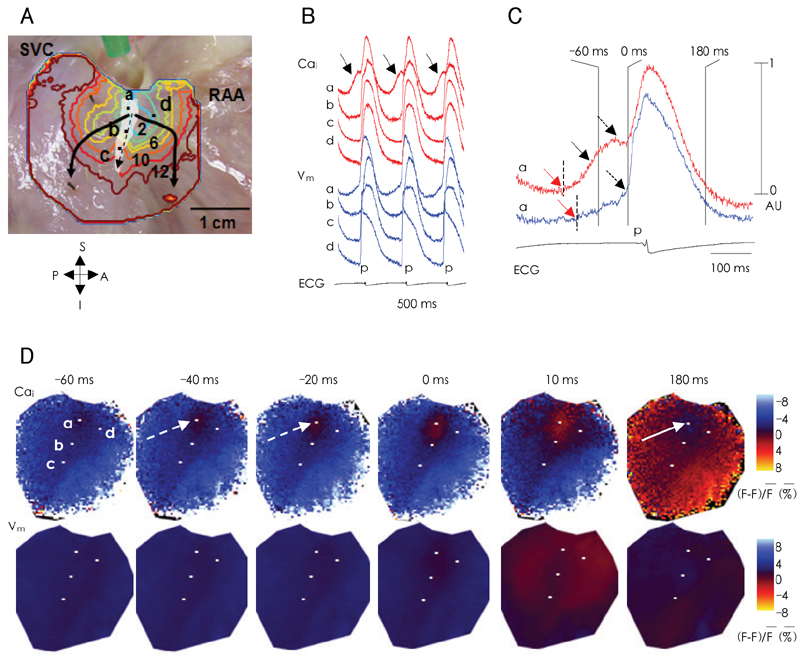
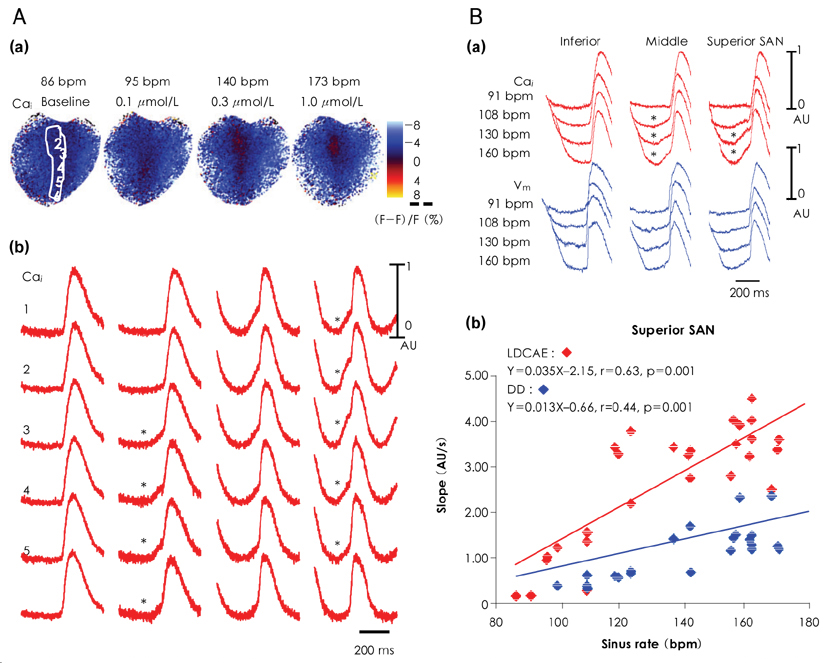
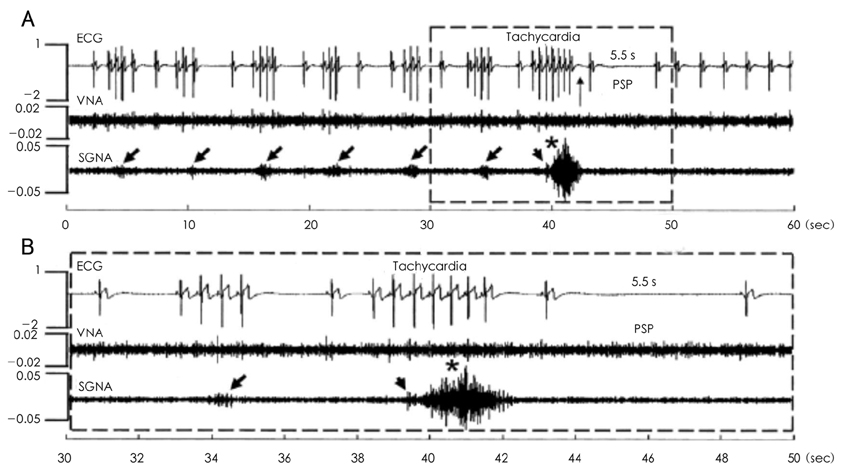
- Recent evidence indicates that the voltage (cyclic activation and deactivation of membrane ion channels) and Ca2+ clocks (rhythmic spontaneous sarcoplasmic reticulum Ca2+ release) jointly regulate sinoatrial node (SAN) automaticity. Since the intact SAN is a heterogeneous structure that includes multiple different cell types interacting with each other, the relative importance of the voltage and Ca2+ clocks for pacemaking may be variable in different regions of the SAN. Recently, we performed optical mapping in isolated and Langendorff-perfused canine right atria. We mapped the intracellular calcium (Cai) and membrane potentials of the intact SAN simultaneously. Using previously described criteria of the timing of the late diastolic Cai elevation (LDCAE) relative to the action potential upstroke to detect Ca2+ clock activity, we demonstrated that the sinus rate increased and the leading pacemaker shifted to the superior SAN with the robust LDCAE during beta-adrenergic stimulation. We also showed that the LDCAE was caused by spontaneous diastolic SR Ca2+ release and was closely related with heart rate changes. We conclude that the Ca2+ and voltage clocks work synergistically to generate SAN automaticity.
MeSH Terms
Figure
Reference
-
1. Adan V, Crown LA. Diagnosis and treatment of sick sinus syndrome. Am Fam Physician. 2003. 67:1725–1732.2. Brown HF, DiFrancesco D, Noble SJ. How does adrenaline accelerate the heart? Nature. 1979. 280:235–236.3. Baruscotti M, Bucchi A, DiFrancesco D. Physiology and pharmacology of the cardiac pacemaker ("funny") current. Pharmacol Ther. 2005. 107:59–79.4. DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and developments. Prog Biophys Mol Biol. 1985. 46:163–183.5. Mangoni ME, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev. 2008. 88:919–982.6. Milanesi R, Baruscotti M, Gnecchi-Ruscone T, DiFrancesco D. Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. N Engl J Med. 2006. 354:151–157.7. Bucchi A, Baruscotti M, DiFrancesco D. Current-dependent block of rabbit sino-atrial node I(f) channels by ivabradine. J Gen Physiol. 2002. 120:1–13.8. DiFrancesco D. Funny channels in the control of cardiac rhythm and mode of action of selective blockers. Pharmacol Res. 2006. 53:399–406.9. Nof E, Luria D, Brass D, et al. Point mutation in the HCN4 cardiac ion channel pore affecting synthesis, trafficking, and functional expression is associated with familial asymptomatic sinus bradycardia. Circulation. 2007. 116:463–470.10. Rubenstein DS, Lipsius SL. Mechanisms of automaticity in subsidiary pacemakers from cat right atrium. Circ Res. 1989. 64:648–657.11. Zhou Z, Lipsius SL. Na(+)-Ca2+ exchange current in latent pacemaker cells isolated from cat right atrium. J Physiol. 1993. 466:263–285.12. Hata T, Noda T, Nishimura M, Watanabe Y. The role of Ca2+ release from sarcoplasmic reticulum in the regulation of sinoatrial node automaticity. Heart Vessels. 1996. 11:234–241.13. Rigg L, Terrar DA. Possible role of calcium release from the sarcoplasmic reticulum in pacemaking in guinea-pig sino-atrial node. Exp Physiol. 1996. 81:877–880.14. Li J, Qu J, Nathan RD. Ionic basis of ryanodine's negative chronotropic effect on pacemaker cells isolated from the sinoatrial node. Am J Physiol. 1997. 273:H2481–H2489.15. Ju YK, Allen DG. Intracellular calcium and Na+-Ca2+ exchange current in isolated toad pacemaker cells. J Physiol. 1998. 508:153–166.16. Huser J, Blatter LA, Lipsius SL. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol. 2000. 524:415–422.17. Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na(+)-Ca(2+) exchanger: molecular partners in pacemaker regulation. Circ Res. 2001. 88:1254–1258.18. Vinogradova TM, Bogdanov KY, Lakatta EG. beta-Adrenergic stimulation modulates ryanodine receptor Ca(2+) release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res. 2002. 90:73–79.19. Vinogradova TM, Bogdanov KY, Lakatta EG. Novel perspectives on the beating rate of the heart. Circ Res. 2002. 91:e3.20. Maltsev VA, Vinogradova TM, Lakatta EG. The emergence of a general theory of the initiation and strength of the heartbeat. J Pharmacol Sci. 2006. 100:338–369.21. Vinogradova TM, Lyashkov AE, Zhu W, et al. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006. 98:505–514.22. Bhuiyan ZA, van den Berg MP, van Tintelen JP, et al. Expanding spectrum of human RYR2-related disease: new electrocardiographic, structural, and genetic features. Circulation. 2007. 116:1569–1576.23. Verheijck EE, van Kempen MJ, Veereschild M, Lurvink J, Jongsma HJ, Bouman LN. Electrophysiological features of the mouse sinoatrial node in relation to connexin distribution. Cardiovasc Res. 2001. 52:40–50.24. Lei M, Jones SA, Liu J, et al. Requirement of neuronal- and cardiac-type sodium channels for murine sinoatrial node pacemaking. J Physiol. 2004. 559:835–848.25. Lancaster MK, Jones SA, Harrison SM, Boyett MR. Intracellular Ca2+ and pacemaking within the rabbit sinoatrial node: heterogeneity of role and control. J Physiol. 2004. 556:481–494.26. Tellez JO, Dobrzynski H, Greener ID, et al. Differential expression of ion channel transcripts in atrial muscle and sinoatrial node in rabbit. Circ Res. 2006. 99:1384–1393.27. Boineau JP, Miller CB, Schuessler RB, et al. Activation sequence and potential distribution maps demonstrating multicentric atrial impulse origin in dogs. Circ Res. 1984. 54:332–347.28. Boineau JP, Schuessler RB, Mooney CR, et al. Multicentric origin of the atrial depolarization wave: the pacemaker complex: relation to dynamics of atrial conduction, P-wave changes and heart rate control. Circulation. 1978. 58:1036–1048.29. Schuessler RB, Boineau JP, Wylds AC, Hill DA, Miller CB, Roeske WR. Effect of canine cardiac nerves on heart rate, rhythm, and pacemaker location. Am J Physiol. 1986. 250:H630–H644.30. Joung B, Tang L, Maruyama M, et al. Intracellular calcium dynamics and the acceleration of sinus rhythm by beta-adrenergic stimulation. Circulation. 2009. 119:788–796.31. Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007. 115:1921–1932.32. Sanders P, Kistler PM, Morton JB, Spence SJ, Kalman JM. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation. 2004. 110:897–903.33. Ogawa M, Zhou S, Tan AY, et al. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 2007. 50:335–343.34. Ogawa M, Tan AY, Song J, et al. Cryoablation of extrinsic cardiac sympathetic nerves markedly reduces atrial arrhythmias in ambulatory dogs with pacing-induced heart failure. Heart Rhythm. 2008. 5:S54. Abstract.35. Yeh YH, Burstein B, Qi XY, et al. Funny current downregulation and sinus node dysfunction associated with atrial tachyarrhythmia: a molecular basis for tachycardia-bradycardia syndrome. Circulation. 2009. 119:1576–1585.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Function and Dysfunction of Human Sinoatrial Node
- The Role of the Calcium and the Voltage Clocks in Sinoatrial Node Dysfunction
- Heart Rate Acceleration of a Subsidiary Pacemaker by beta-Adrenergic Stimulation
- The Gradient Model of the Rabbit Sinoatrial Node
- RE: An Unusual Course of Right Coronary Artery Originating from Sinoatrial Node Artery