Korean Circ J.
2011 Nov;41(11):658-665. 10.4070/kcj.2011.41.11.658.
Heart Rate Acceleration of a Subsidiary Pacemaker by beta-Adrenergic Stimulation
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. cby6908@yuhs.ac
- 2Division of Cardiology, Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- KMID: 2225074
- DOI: http://doi.org/10.4070/kcj.2011.41.11.658
Abstract
- BACKGROUND AND OBJECTIVES
Recent evidence indicates that the membrane voltage and Ca2+ clocks jointly regulate sinoatrial node (SAN) automaticity. However, the mechanism of heart rhythm acceleration of the subsidiary pacemaker (SP) during beta-adrenergic stimulation is still unknown. Here we tested the hypothesis that the heart rate acceleration of the SP by beta-adrenergic stimulation involves synergistic interactions between both clock mechanisms.
SUBJECTS AND METHODS
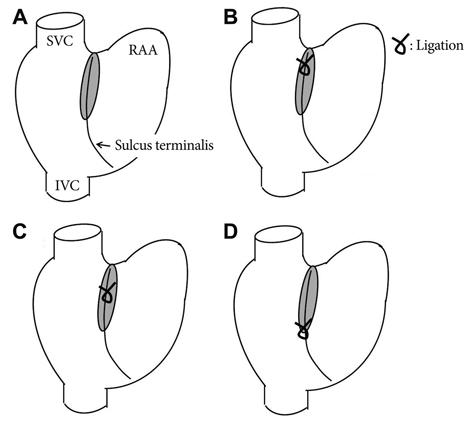
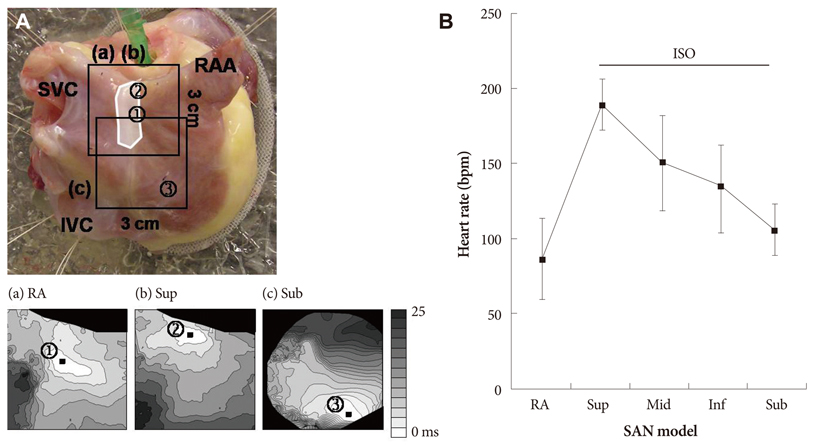
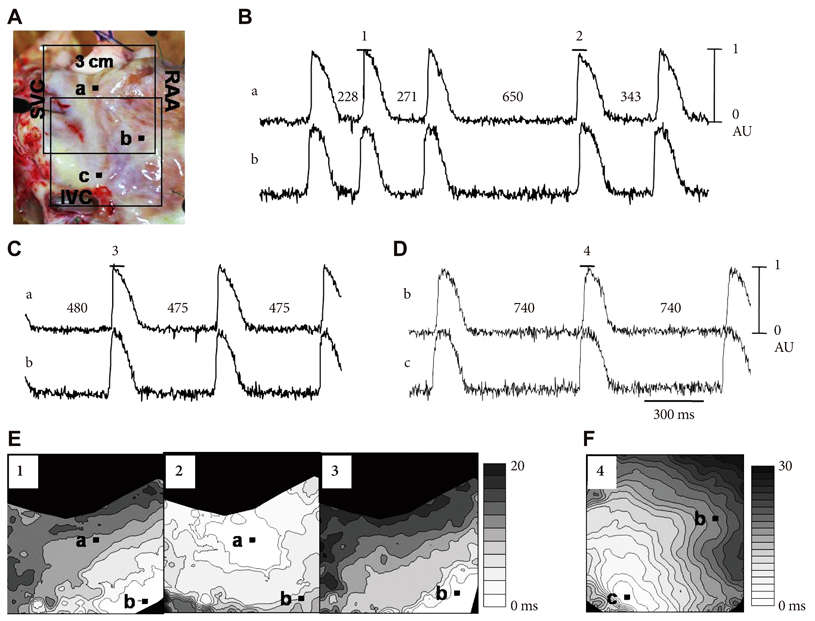
We performed optical mapping and pharmacological interventions in 15 isolated Langendorff-perfused canine right atriums (RA). The SP model was produced by ligation of the SAN artery at the mid portion of the sulcus terminalis.
RESULTS
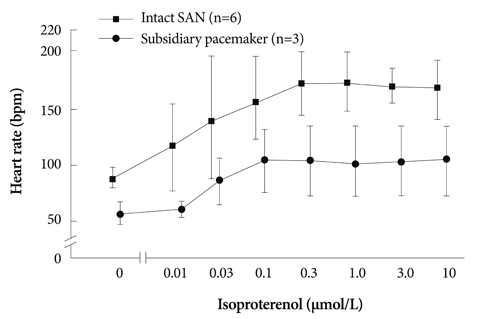
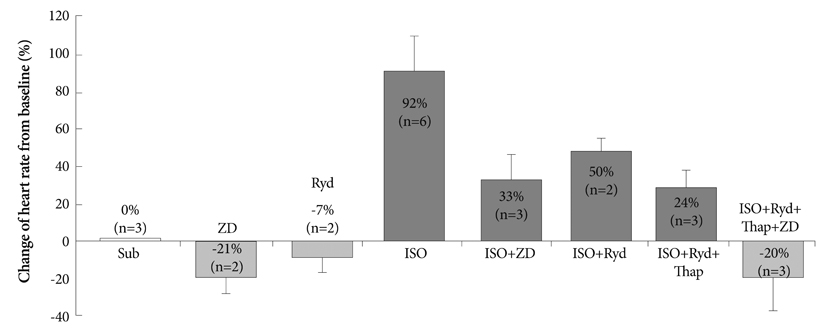
In the 6 RAs with an intact SAN, 1 micromol/L isoproterenol infusion increased the heart rate from 82+/-9 to 166+/-18 bpm (102%) with late diastolic Cai elevation (LDCAE) at the superior SAN. However, in the 6 SP models, the heart rate increased from 55+/-10 bpm to 106+/-11 bpm (92%, p=0.005) without LDCAE at the earliest activation site. The isoproterenol induced heart rate increase was reversed to 74+/-5 bpm (33% from baseline) by administering an infusion of the funny current blocker ZD 7288 (3 micromol/L, n=3), whereas, it was suppressed to 69+/-7 bpm (24% from baseline) by sarcoplasmic reticulum (SR) Ca2+ emptying with administering ryanodine (10 micromol/L) plus thapsigargin (200 nmol/L, n=3). The isoproterenol induced heart rate increase was completely abolished by combined treatment with funny current blocker and SR Ca2+ emptying (n=3).
CONCLUSION
Acceleration of the Ca2+ clock in the SP plays an important role in the heart rate acceleration during beta-adrenergic stimulation, and this interacts synergistically with the voltage clock to increase the heart rate.
MeSH Terms
Figure
Reference
-
1. Adán V, Crown LA. Diagnosis and treatment of sick sinus syndrome. Am Fam Physician. 2003. 67:1725–1732.2. Randall WC, Talano J, Kaye MP, Euler D, Jones S, Brynjolfsson G. Cardiac pacemakers in absence of the SA node: responses to exercise and autonomic blockade. Am J Physiol. 1978. 234:H465–H470.3. Rozanski GJ, Lipsius SL, Randall WC. Functional characteristics of sinoatrial and subsidiary pacemaker activity in the canine right atrium. Circulation. 1983. 67:1378–1387.4. DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993. 55:455–472.5. DiFrancesco D. The pacemaker current (I(f)) plays an important role in regulating SA node pacemaker activity. Cardiovasc Res. 1995. 30:307–308.6. Hata T, Noda T, Nishimura M, Watanabe Y. The role of Ca2+ release from sarcoplasmic reticulum in the regulation of sinoatrial node automaticity. Heart Vessels. 1996. 11:234–241.7. Ju YK, Allen DG. Intracellular calcium and Na+-Ca2+ exchange current in isolated toad pacemaker cells. J Physiol. 1998. 508:153–166.8. Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na(+)-Ca(2+) exchanger: molecular partners in pacemaker regulation. Circ Res. 2001. 88:1254–1258.9. Vinogradova TM, Bogdanov KY, Lakatta EG. Beta-adrenergic stimulation modulates ryanodine receptor Ca(2+) release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res. 2002. 90:73–79.10. Vinogradova TM, Zhou YY, Maltsev V, Lyashkov A, Stern M, Lakatta EG. Rhythmic ryanodine receptor Ca2+ releases during diastolic depolarization of sinoatrial pacemaker cells do not require membrane depolarization. Circ Res. 2004. 94:802–809.11. Vinogradova TM, Lyashkov AE, Zhu W, et al. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006. 98:505–514.12. Joung B, Tang L, Maruyama M, et al. Intracellular calcium dynamics and acceleration of sinus rhythm by beta-adrenergic stimulation. Circulation. 2009. 119:788–796.13. Joung B, Ogawa M, Lin SF, Chen PS. The calcium and voltage clocks in sinoatrial node automaticity. Korean Circ J. 2009. 39:217–222.14. Joung B, Shinohara T, Zhang H, et al. Tachybradycardia in the isolated canine right atrium induced by chronic sympathetic stimulation and pacemaker current inhibition. Am J Physiol Heart Circ Physiol. 2010. 299:H634–H642.15. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias: the Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997. 337:1576–1583.16. Joung BY, Koo BK, Xu ZZ, Kim IK, Lee MH, Kim US. The effects of obstacles on the dynamics of ventricular fibrillation. Korean Circ J. 2005. 35:183–191.17. Fedorov VV, Schuessler RB, Hemphill M, et al. Structural and functional evidence for discrete exit pathways that connect the canine sinoatrial node and atria. Circ Res. 2009. 104:915–923.18. Efimov IR, Fedorov VV, Joung B, Lin SF. Mapping cardiac pacemaker circuits: methodological puzzles of the sinoatrial node optical mapping. Circ Res. 2010. 106:255–271.19. Boineau JP, Schuessler RB, Mooney CR, et al. Multicentric origin of the atrial depolarization wave: the pacemaker complex: relation to dynamics of atrial conduction, P-wave changes and heart rate control. Circulation. 1978. 58:1036–1048.20. Boineau JP, Miller CB, Schuessler RB, et al. Activation sequence and potential distribution maps demonstrating multicentric atrial impulse origin in dogs. Circ Res. 1984. 54:332–347.21. Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007. 115:1921–1932.22. Rubenstein DS, Lipsius SL. Mechanisms of automaticity in subsidiary pacemakers from cat right atrium. Circ Res. 1989. 64:648–657.23. Verheijck EE, vanKempen MJ, Veereschild M, Lurvink J, Jongsma HJ, Bouman LN. Electrophysiological features of the mouse sinoatrial node in relation to connexin distribution. Cardiovasc Res. 2001. 52:40–50.24. Lei M, Jones SA, Liu J, et al. Requirement of neuronal- and cardiac-type sodium channels for murine sinoatrial node pacemaking. J Physiol. 2004. 559:835–848.25. Lancaster MK, Jones SA, Harrison SM, Boyett MR. Intracellular Ca2+ and pacemaking within the rabbit sinoatrial node: heterogeneity of role and control. J Physiol. 2004. 556:481–494.26. Tellez JO, Dobrzynski H, Greener ID, et al. Differential expression of ion channel transcripts in atrial muscle and sinoatrial node in rabbit. Circ Res. 2006. 99:1384–1393.27. Efimov IR, Nikolski VP, Salama G. Optical imaging of the heart. Circ Res. 2004. 95:21–33.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characteristics of Subsidiary Pacemaker in Complete Heartblock with Narrow QRS Complex
- Alterations of beta-Adrenergic Receptor Signaling in Cardiac Hypertrophy and Heart Failure: beta-Adrenergic Receptor Desensitization in Cardiac Disease
- The Effect of Beta-Adrenergic Receptor Blockade on the Atrial Refractory Period of Hyperthyroid Rabbits
- Function and Dysfunction of Human Sinoatrial Node
- A Case of Pacemaker Implantation in Premature Newborn with Congenital Complete Atrioventricular Block