Korean Circ J.
2015 May;45(3):184-191. 10.4070/kcj.2015.45.3.184.
Function and Dysfunction of Human Sinoatrial Node
- Affiliations
-
- 1Division of Cardiology, Department of Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 2The Krannert Institute of Cardiology and the Division of Cardiology, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA. chenpp@iupui.edu
- KMID: 2389124
- DOI: http://doi.org/10.4070/kcj.2015.45.3.184
Abstract
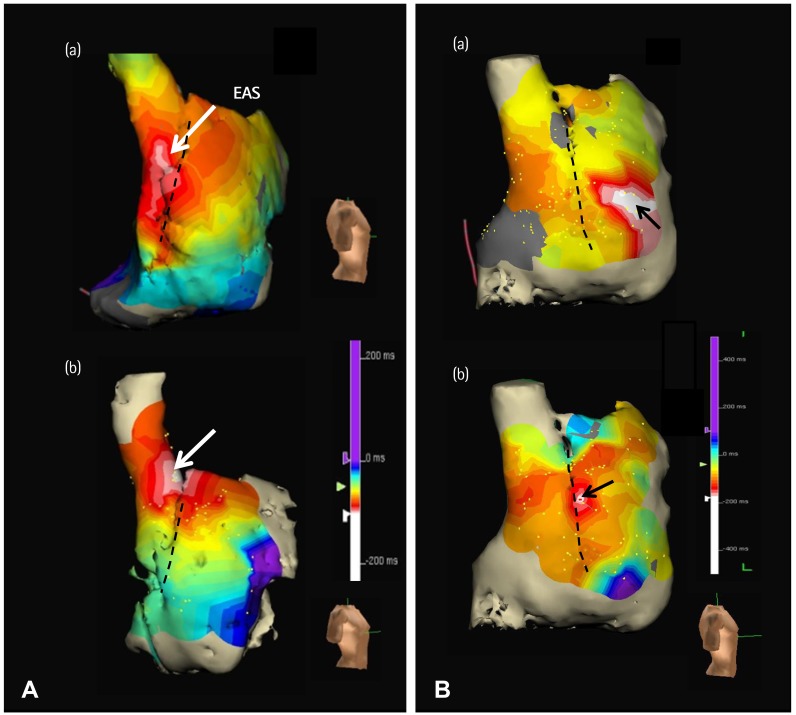
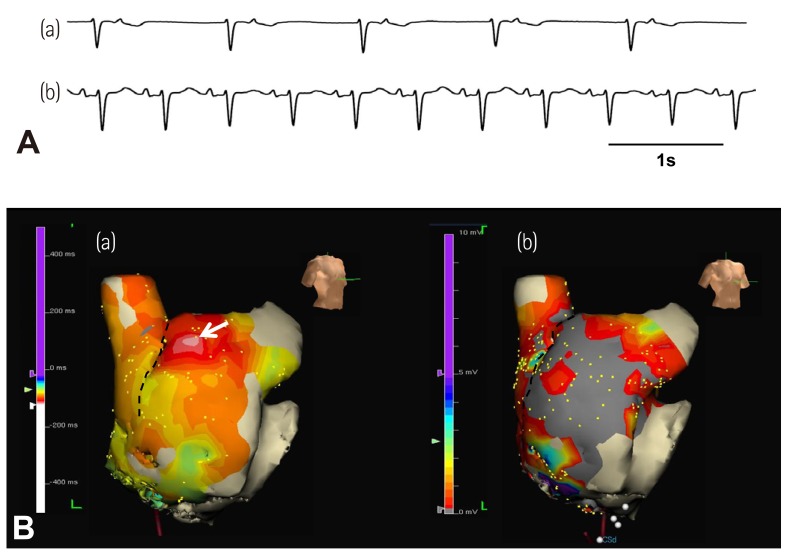
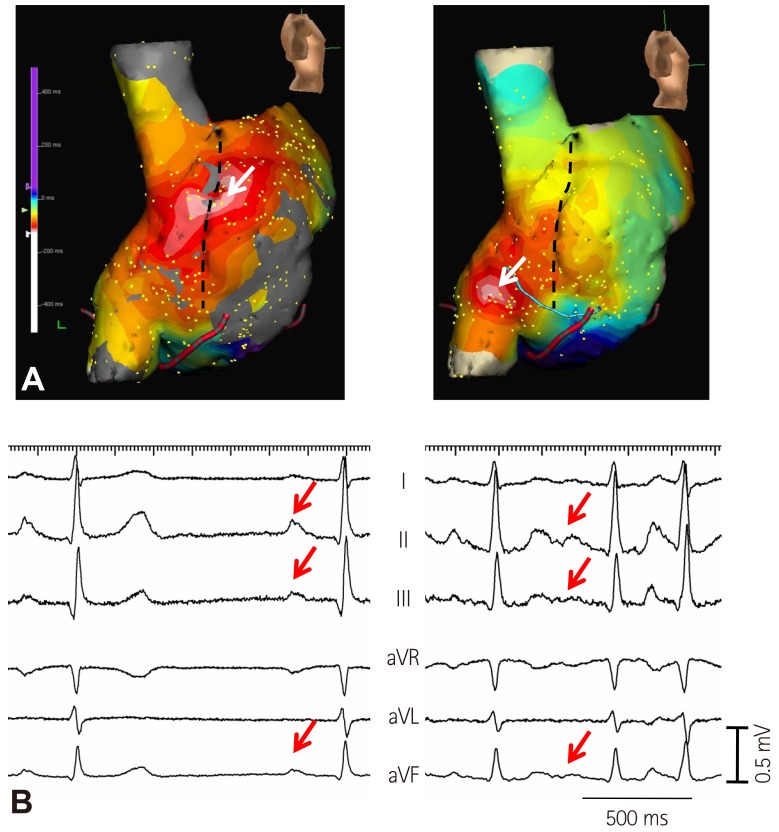
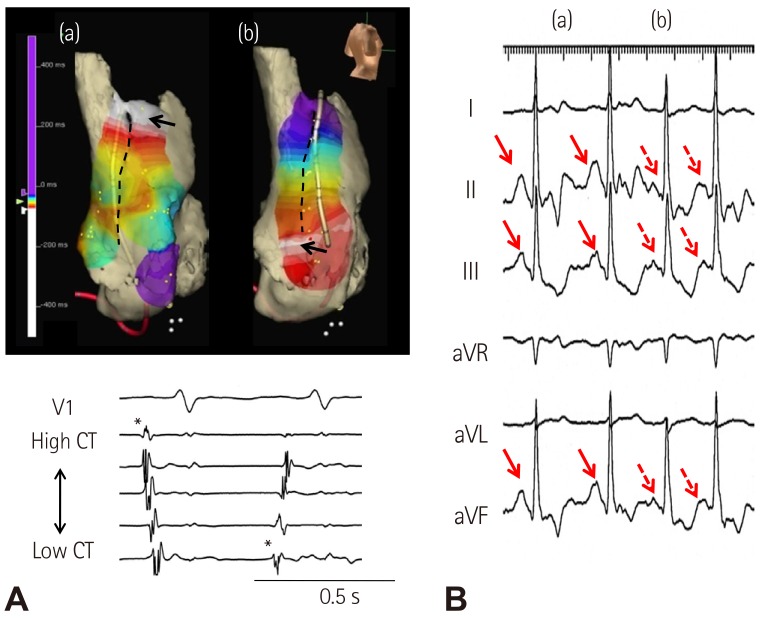
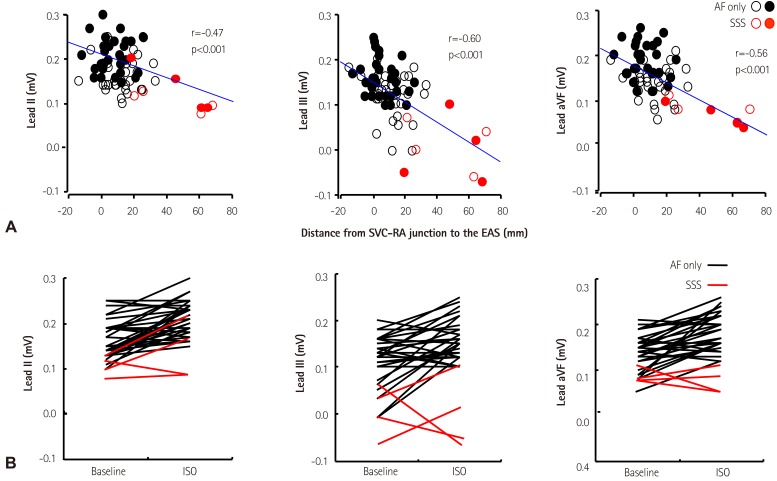
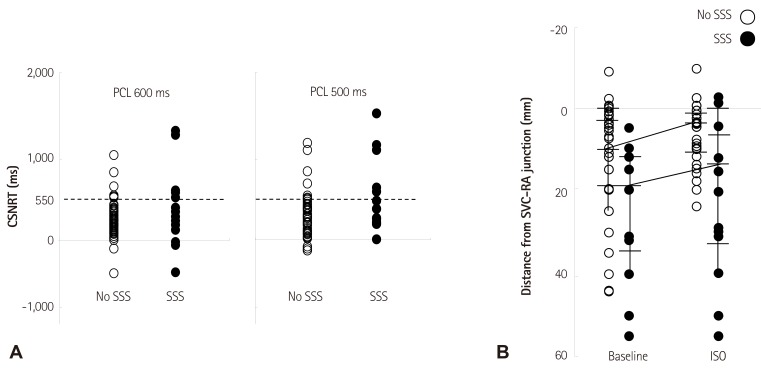
- Sinoatrial node (SAN) automaticity is jointly regulated by a voltage (cyclic activation and deactivation of membrane ion channels) and Ca2+ clocks (rhythmic spontaneous sarcoplasmic reticulum Ca2+ release). Using optical mapping in Langendorff-perfused canine right atrium, we previously demonstrated that the beta-adrenergic stimulation pushes the leading pacemaker to the superior SAN, which has the fastest activation rate and the most robust late diastolic intracellular calcium (Cai) elevation. Dysfunction of the superior SAN is commonly observed in animal models of heart failure and atrial fibrillation (AF), which are known to be associated with abnormal SAN automaticity. Using the 3D electroanatomic mapping techniques, we demonstrated that superior SAN served as the earliest atrial activation site (EAS) during sympathetic stimulation in healthy humans. In contrast, unresponsiveness of superior SAN to sympathetic stimulation was a characteristic finding in patients with AF and SAN dysfunction, and the 3D electroanatomic mapping technique had better diagnostic sensitivity than corrected SAN recovery time testing. However, both tests have significant limitations in detecting patients with symptomatic sick sinus syndrome. Recently, we reported that the location of the EAS can be predicted by the amplitudes of P-wave in the inferior leads. The inferior P-wave amplitudes can also be used to assess the superior SAN responsiveness to sympathetic stimulation. Inverted or isoelectric P-waves at baseline that fail to normalize during isoproterenol infusion suggest SAN dysfunction. P-wave morphology analyses may be helpful in determining the SAN function in patients at risk of symptomatic sick sinus syndrome.
Keyword
MeSH Terms
Figure
Reference
-
1. Adán V, Crown LA. Diagnosis and treatment of sick sinus syndrome. Am Fam Physician. 2003; 67:1725–1732. PMID: 12725451.2. DiFrancesco D. The role of the funny current in pacemaker activity. Circ Res. 2010; 106:434–446. PMID: 20167941.3. Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker. Circ Res. 2010; 106:659–673. PMID: 20203315.4. Verheijck EE, van Kempen MJ, Veereschild M, Lurvink J, Jongsma HJ, Bouman LN. Electrophysiological features of the mouse sinoatrial node in relation to connexin distribution. Cardiovasc Res. 2001; 52:40–50. PMID: 11557232.5. Lancaster MK, Jones SA, Harrison SM, Boyett MR. Intracellular Ca2+ and pacemaking within the rabbit sinoatrial node: heterogeneity of role and control. J Physiol. 2004; 556:481–494. PMID: 14724216.6. Tellez JO, Dobrzynski H, Greener ID, et al. Differential expression of ion channel transcripts in atrial muscle and sinoatrial node in rabbit. Circ Res. 2006; 99:1384–1393. PMID: 17082478.7. Joung B, Tang L, Maruyama M, et al. Intracellular calcium dynamics and acceleration of sinus rhythm by beta-adrenergic stimulation. Circulation. 2009; 119:788–796. PMID: 19188501.8. Joung B, Chen PS, Lin SF. The role of the calcium and the voltage clocks in sinoatrial node dysfunction. Yonsei Med J. 2011; 52:211–219. PMID: 21319337.9. Chen PS, Joung B, Shinohara T, Das M, Chen Z, Lin SF. The initiation of the heart beat. Circ J. 2010; 74:221–225. PMID: 20019407.10. Joung B, Ogawa M, Lin SF, Chen PS. The calcium and voltage clocks in sinoatrial node automaticity. Korean Circ J. 2009; 39:217–222. PMID: 19949626.11. Efimov IR, Fedorov VV, Joung B, Lin SF. Mapping cardiac pacemaker circuits: methodological puzzles of the sinoatrial node optical mapping. Circ Res. 2010; 106:255–271. PMID: 20133911.12. Boineau JP, Miller CB, Schuessler RB, et al. Activation sequence and potential distribution maps demonstrating multicentric atrial impulse origin in dogs. Circ Res. 1984; 54:332–347. PMID: 6697451.13. Boineau JP, Schuessler RB, Mooney CR, et al. Multicentric origin of the atrial depolarization wave: the pacemaker complex. Relation to dynamics of atrial conduction, P-wave changes and heart rate control. Circulation. 1978; 58:1036–1048. PMID: 709760.14. Schuessler RB, Boineau JP, Wylds AC, Hill DA, Miller CB, Roeske WR. Effect of canine cardiac nerves on heart rate, rhythm, and pacemaker location. Am J Physiol. 1986; 250:H630–H644. PMID: 3963219.15. Li J, Qu J, Nathan RD. Ionic basis of ryanodine's negative chronotropic effect on pacemaker cells isolated from the sinoatrial node. Am J Physiol. 1997; 273:H2481–H2489. PMID: 9374788.16. Ju YK, Allen DG. Intracellular calcium and Na+-Ca2+ exchange current in isolated toad pacemaker cells. J Physiol. 1998; 508:153–166. PMID: 9490832.17. Hüser J, Blatter LA, Lipsius SL. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol. 2000; 524(Pt 2):415–422. PMID: 10766922.18. Vinogradova TM, Bogdanov KY, Lakatta EG. Beta-adrenergic stimulation modulates ryanodine receptor Ca(2+) release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res. 2002; 90:73–79. PMID: 11786521.19. Maltsev VA, Vinogradova TM, Lakatta EG. The emergence of a general theory of the initiation and strength of the heartbeat. J Pharmacol Sci. 2006; 100:338–369. PMID: 16799255.20. Vinogradova TM, Lyashkov AE, Zhu W, et al. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006; 98:505–514. PMID: 16424365.21. Park S, Park H, Hwang HJ, et al. Heart rate acceleration of a subsidiary pacemaker by β-adrenergic stimulation. Korean Circ J. 2011; 41:658–665. PMID: 22194761.22. Kim D, Shinohara T, Joung B, et al. Calcium dynamics and the mechanisms of atrioventricular junctional rhythm. J Am Coll Cardiol. 2010; 56:805–812. PMID: 20797495.23. Joung B, Zhang H, Shinohara T, et al. Delayed afterdepolarization in intact canine sinoatrial node as a novel mechanism for atrial arrhythmia. J Cardiovasc Electrophysiol. 2011; 22:448–454. PMID: 21040091.24. Joung B, Shinohara T, Zhang H, et al. Tachybradycardia in the isolated canine right atrium induced by chronic sympathetic stimulation and pacemaker current inhibition. Am J Physiol Heart Circ Physiol. 2010; 299:H634–H642. PMID: 20601460.25. Marrouche NF, Beheiry S, Tomassoni G, et al. Three-dimensional nonfluoroscopic mapping and ablation of inappropriate sinus tachycardia. Procedural strategies and long-term outcome. J Am Coll Cardiol. 2002; 39:1046–1054. PMID: 11897449.26. Joung B, Hwang HJ, Pak HN, et al. Abnormal response of superior sinoatrial node to sympathetic stimulation is a characteristic finding in patients with atrial fibrillation and symptomatic bradycardia. Circ Arrhythm Electrophysiol. 2011; 4:799–807. PMID: 22007035.27. Gomes JA, Kang PS, Matheson M, Gough WB Jr, El-Sherif N. Coexistence of sick sinus rhythm and atrial flutter-fibrillation. Circulation. 1981; 63:80–86. PMID: 7438410.28. van den Berg MP, van Gelder IC. Atrial fibrillation and sinus node dysfunction. J Am Coll Cardiol. 2001; 38:1585–1586. PMID: 11691547.29. Hocini M, Sanders P, Deisenhofer I, et al. Reverse remodeling of sinus node function after catheter ablation of atrial fibrillation in patients with prolonged sinus pauses. Circulation. 2003; 108:1172–1175. PMID: 12952840.30. Elvan A, Wylie K, Zipes DP. Pacing-induced chronic atrial fibrillation impairs sinus node function in dogs. Electrophysiological remodeling. Circulation. 1996; 94:2953–2960. PMID: 8941126.31. Joung B, Lin SF, Chen Z, et al. Mechanisms of sinoatrial node dysfunction in a canine model of pacing-induced atrial fibrillation. Heart Rhythm. 2010; 7:88–95. PMID: 19914141.32. Shinohara T, Park HW, Han S, et al. Ca2+ clock malfunction in a canine model of pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 2010; 299:H1805–H1811. PMID: 20889842.33. Fedorov VV, Schuessler RB, Hemphill M, et al. Structural and functional evidence for discrete exit pathways that connect the canine sinoatrial node and atria. Circ Res. 2009; 104:915–923. PMID: 19246679.34. Aronow WS. Management of the older person with atrial fibrillation. J Am Geriatr Soc. 1999; 47:740–748. PMID: 10366178.35. Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001; 37:371–378. PMID: 11216949.36. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001; 285:2370–2375. PMID: 11343485.37. Moon J, Hong YJ, Shim J, et al. Right atrial anatomical remodeling affects early outcomes of nonvalvular atrial fibrillation after radiofrequency ablation. Circ J. 2012; 76:860–867. PMID: 22293450.38. Essebag V, Hadjis T, Platt RW, Pilote L. Amiodarone and the risk of bradyarrhythmia requiring permanent pacemaker in elderly patients with atrial fibrillation and prior myocardial infarction. J Am Coll Cardiol. 2003; 41:249–254. PMID: 12535818.39. Mun HS, Shen C, Pak HN, et al. Chronic amiodarone therapy impairs the function of the superior sinoatrial node in patients with atrial fibrillation. Circ J. 2013; 77:2255–2263. PMID: 23739532.40. Kodama I, Kamiya K, Toyama J. Cellular electropharmacology of amiodarone. Cardiovasc Res. 1997; 35:13–29. PMID: 9302343.41. Maltsev VA, Lakatta EG. Dynamic interactions of an intracellular Ca2+ clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function. Cardiovasc Res. 2008; 77:274–284. PMID: 18006441.42. Turker I, Yu CC, Chang PC, et al. Amiodarone inhibits apamin-sensitive potassium currents. PLoS One. 2013; 8:e70450. PMID: 23922993.43. Chen WT, Chen YC, Lu YY, et al. Apamin modulates electrophysiological characteristics of the pulmonary vein and the Sinoatrial Node. Eur J Clin Invest. 2013; 43:957–963. PMID: 23834267.44. Zhang Q, Timofeyev V, Lu L, et al. Functional roles of a Ca2+-activated K+ channel in atrioventricular nodes. Circ Res. 2008; 102:465–471. PMID: 18096820.45. Sanders P, Kistler PM, Morton JB, Spence SJ, Kalman JM. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation. 2004; 110:897–903. PMID: 15302799.46. Kistler PM, Roberts-Thomson KC, Haqqani HM, et al. P-wave morphology in focal atrial tachycardia: development of an algorithm to predict the anatomic site of origin. J Am Coll Cardiol. 2006; 48:1010–1017. PMID: 16949495.47. Uhm JS, Shim J, Wi J, et al. An electrocardiography algorithm combined with clinical features could localize the origins of focal atrial tachycardias in adjacent structures. Europace. 2014; 16:1061–1068. PMID: 24381331.48. Wu Y, Rasmussen TP, Koval OM, et al. The mitochondrial uniporter controls fight or flight heart rate increases. Nat Commun. 2015; 6:6081. PMID: 25603276.49. Wu Y, Gao Z, Chen B, et al. Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proc Natl Acad Sci U S A. 2009; 106:5972–5977. PMID: 19276108.50. Swaminathan PD, Purohit A, Soni S, Voigt N, et al. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest. 2011; 121:3277–3288. PMID: 21785215.51. Huke S, Knollmann BC. Oxidized CaMKII: a "heart stopper" for the sinus node? J Clin Invest. 2011; 121:2975–2977. PMID: 21785211.52. Lou Q, Hansen BJ, Fedorenko O, et al. Upregulation of adenosine A1 receptors facilitates sinoatrial node dysfunction in chronic canine heart failure by exacerbating nodal conduction abnormalities revealed by novel dual-sided intramural optical mapping. Circulation. 2014; 130:315–324. PMID: 24838362.53. Luo M, Guan X, Luczak ED, et al. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest. 2013; 123:1262–1274. PMID: 23426181.54. Wolf RM, Glynn P, Hashemi S, et al. Atrial fibrillation and sinus node dysfunction in human ankyrin-B syndrome: a computational analysis. Am J Physiol Heart Circ Physiol. 2013; 304:H1253–H1266. PMID: 23436330.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Gradient Model of the Rabbit Sinoatrial Node
- RE: An Unusual Course of Right Coronary Artery Originating from Sinoatrial Node Artery
- Sinus Node Dysfunction with Pulmonary Edema Associated with Hyponatremia
- Clinical Electrophysiological Study on Sick Sinus Syndrome
- Diagnosis of sick sinus syndrome with intravenous adenosine injection