Clin Exp Otorhinolaryngol.
2009 Mar;2(1):20-27. 10.3342/ceo.2009.2.1.20.
Effect of Botulinum Toxin Type A on a Rat Surgical Wound Model
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Pusan National University School of Medicine, Korea and the Medical Research Institute, Pusan National University, Busan, Korea. gohek@pusan.ac.kr
- 2Department of Pathology, Pusan National University School of Medicine, Korea and the Medical Research Institute, Pusan National University, Busan, Korea.
- KMID: 1466558
- DOI: http://doi.org/10.3342/ceo.2009.2.1.20
Abstract
- \
OBJECTIVES
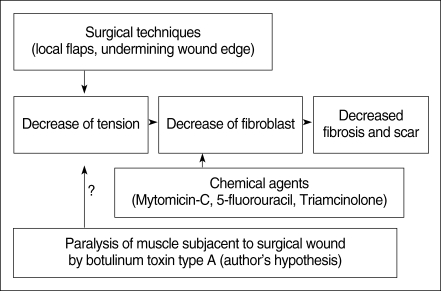
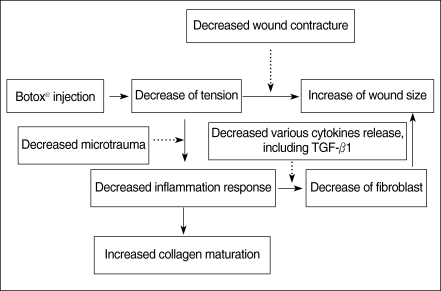
The tension on a wound is one of the important factors that determine the degree of fibrosis and scar formation. We hypothesized that local botulinum toxin type A (Botox) induced paralysis of the musculature subjacent to a surgical wound with a skin defect would minimize the repetitive tensile forces on the surgical wound's edges, and this will result in a decreased fibroplastic response and fibrosis of the wound.
METHODS
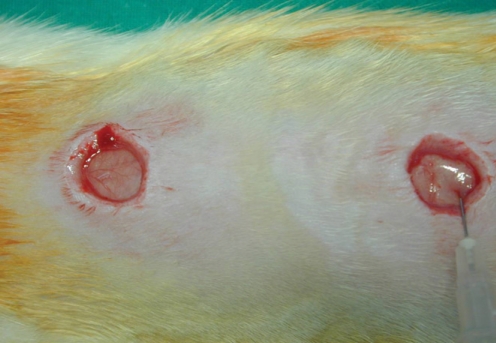
This is a prospective randomized experimental study. Two distinct surgical wounds were made to the dorsum of 15 adult rats, respectively. One of the 2 wounds was injected with Botox, and the other wound was used as a control, and this was done for all the rats' wounds. We evaluated the wound size, the degree of fibrosis and inflammation, the blood vessel proliferation, the thickness of the wound and the expression of transforming growth factor (TGF)-beta1 in the wounds.
RESULTS
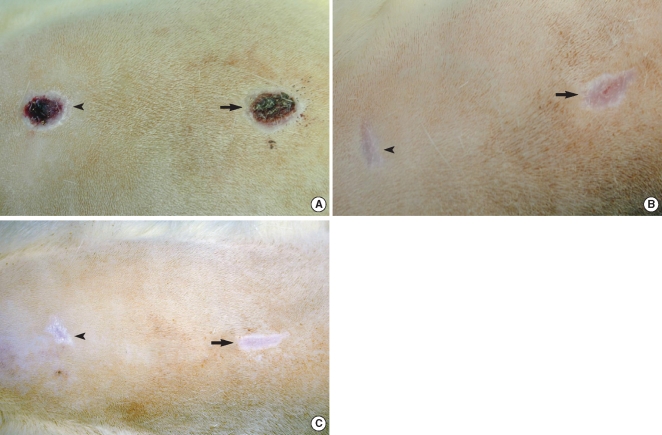
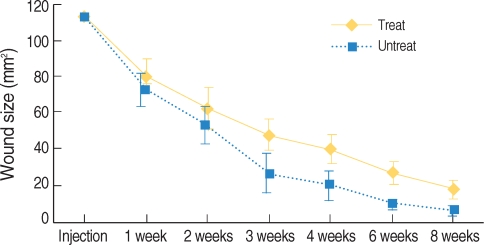
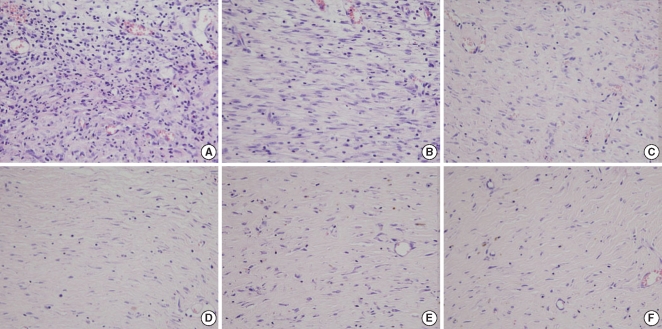
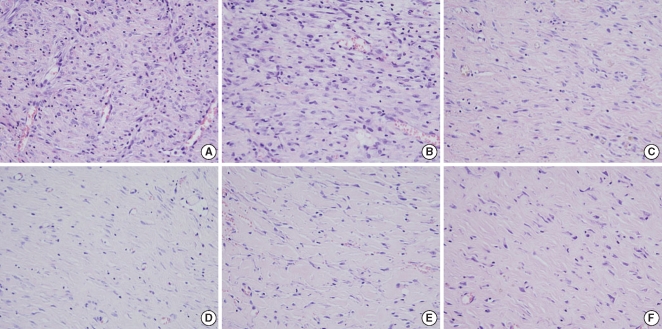
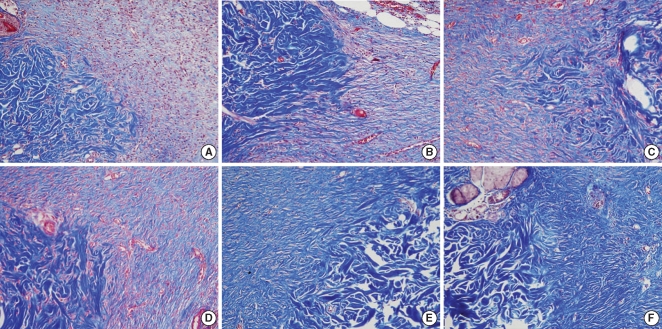
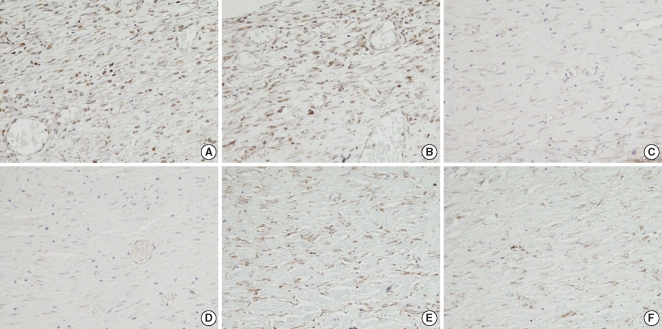
There were significant differences of wound size at the 3rd and 4th week between the Botox and control groups (P<0.05). The Botox group showed less infiltration of inflammatory cells than the control group at the 2nd week (P<0.05). The Botox group showed a smaller number of fibroblasts and less fibrosis than the control group at the 4th week (P<0.05). The Botox group showed much strong collagen density than the control group at the 8th week (P<0.05). For the immunohistochemical staining, there was a lower transforming growth factor (TGF)-beta1 expression in the Botox group than that of the control group at the 4th week (P<0.05).
CONCLUSION
The wounds of the Botox-treated group showed a larger wound size, less infiltration of inflammatory cells and less fibrosis, a much greater amount of collagen and a lower expression of TGF-beta1 than did the control group. Botox might be used to decrease the fibrosis of a surgical wound without damaging the epithelial growth in situations for which decreased fibrosis is necessary, such as for treating laryngeal, tracheal and nasal stenosis.
Keyword
MeSH Terms
-
Adult
Animals
Blood Vessels
Botulinum Toxins
Botulinum Toxins, Type A
Cicatrix
Collagen
Constriction, Pathologic
Fibroblasts
Fibrosis
Glycosaminoglycans
Humans
Inflammation
Paralysis
Prospective Studies
Rats
Skin
Transforming Growth Factor beta1
Transforming Growth Factors
Wound Healing
Botulinum Toxins
Botulinum Toxins, Type A
Collagen
Glycosaminoglycans
Transforming Growth Factor beta1
Transforming Growth Factors
Figure
Reference
-
1. Manuskiatti W, Fitzpatrick RE. Treatment response of keloidal and hypertrophic sternotomy scars: comparison among intralesional corticosteroid, 5-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatments. Arch Dermatol. 2002; 9. 138(9):1149–1155. PMID: 12224975.2. Fitzpatrick RE. Treatment of inflamed hypertrophic scars using intralesional 5-FU. Dermatol Surg. 1999; 3. 25(3):224–232. PMID: 10193972.
Article3. Chowdri NA, Masarat M, Mattoo A, Darzi MA. Keloids and hypertrophic scars: results with intraoperative and serial postoperative corticosteroid injection therapy. Aust N Z J Surg. 1999; 9. 69(9):655–659. PMID: 10515339.
Article4. Alster T. Laser scar revision: comparison study of 585-nm pulsed dye laser with and without intralesional corticosteroids. Dermatol Surg. 2003; 1. 29(1):25–29. PMID: 12534508.
Article5. Crooke ST, Bradner WT. Mitomycin C: a review. Cancer Treat Rev. 1976; 9. 3(3):121–139. PMID: 786455.
Article6. Miyamura S, Shigeno N, Matsui M, Wakaki S, Uzu K. The biological studies on mitomycin I: antibacterial activities of mitomycin derivatives. J Antibiot (Tokyo). 1967; 3. 20(2):72–76. PMID: 4964076.7. Hu D, Sires BS, Tong DC, Royack GA, Oda D. Effect of brief exposure to mitomycin C on cultured human nasal mucosa fibroblasts. Ophthal Plast Reconstr Surg. 2000; 3. 16(2):119–125.
Article8. Garrett CG, Soto J, Riddick J, Billante CR, Reinisch L. Effect of mitomycin-C on vocal fold healing in a canine model. Ann Otol Rhinol Laryngol. 2001; 1. 110(1):25–30. PMID: 11201804.
Article9. De Bruijn EA, Sleeboom HP, van Helsdingen PJ, van Oosterom AT, Tjaden UR, Maes RA. Pharmacodynamics and pharmacokinetics of intravesical mitomycin C upon different dwelling times. Int J Cancer. 1992; 5. 28. 51(3):359–364. PMID: 1592527.
Article10. Oboshi S, Matsui M, Ishii S, Masago N, Wakaki S. Antitumor studies on mitomycin derivatives. II. Effec of solid tumor of sarcoma-180. Gann. 1967; 8. 58(4):315–321. PMID: 6079789.11. Hueman EM, Simpson CB. Airway complications from topical mitomycin C. Otolaryngol Head Neck Surg. 2005; 12. 133(6):831–835. PMID: 16360498.
Article12. Sherris DA, Larrabee WF Jr, Murakami CS. Management of scar contractures, hypertrophic scars, and keloids. Otolaryngol Clin North Am. 1995; 10. 28(5):1057–1068. PMID: 8559572.
Article13. Blitzer A, Sulica L. Botulinum toxin: basic science and clinical uses in otolaryngology. Laryngoscope. 2001; 2. 111(2):218–226. PMID: 11210864.
Article14. Keir J. Botulinum toxin-physiology and applications in head and neck disorders. Head Neck. 2005; 6. 27(6):525–535. PMID: 15898076.
Article15. Gassner HG, Sherris DA. Chemoimmobilization: improving predictability in the treatment of facial scars. Plast Reconstr Surg. 2003; 10. 112(5):1464–1466. PMID: 14504533.
Article16. Kantor J, Margolis DJ. Efficacy and prognostic value of simple wound measurements. Arch Dermatol. 1998; 12. 134(12):1571–1574. PMID: 9875195.
Article17. Philips LG. Townsend CM, Beauchamp RD, Evers BM, Mattox KL, editors. Wound healing. Textbook of surgery. 2001. 16th ed. Philadelphia (PA): WB Saunders Co.;p. 131–155.18. Ribeiro Fde A, Guaraldo L, Borges Jde P, Zacchi FF, Eckley CA. Clinical and histological healing of surgical wounds treated with mitomycin C. Laryngoscope. 2004; 1. 114(1):148–152. PMID: 14710012.
Article19. Gassner HG, Sherris DA, Otley CC. Treatment of facial wounds with botulinum toxin A improves cosmetic outcome in primates. Plast Reconstr Surg. 2000; 5. 105(6):1948–1953. PMID: 10839391.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A clinical study on the use of botulinum toxin type a in maxillofacial area
- Application of Botulinum Toxin in Pain Management
- Botulinum Toxin-Type A in Cervical Myofascial Pain Syndrome: A report of 3 cases
- Treatment of Winkles and Hyperhidrosis with Botulinum Toxin Type A
- A CASE REPORT OF TREATMENT OF MASSETERIC HYPERTROPHY WITH BOTULINUM TOXIN