Korean J Physiol Pharmacol.
2013 Apr;17(2):169-173. 10.4196/kjpp.2013.17.2.169.
Oxytocin Ameliorates Remote Liver Injury Induced by Renal Ischemia-Reperfusion in Rats
- Affiliations
-
- 1Department of Pharmacology, Faculty of Medicine, Dicle University, 21280 Diyarbakir, Turkey. hakkoc@dicle.edu.tr
- 2Department of Clinical Biochemistry, Faculty of Medicine, Dicle University, 21280 Diyarbakir, Turkey.
- 3Department of Pathology, Faculty of Medicine, Dicle University, 21280 Diyarbakir, Turkey.
- KMID: 1429389
- DOI: http://doi.org/10.4196/kjpp.2013.17.2.169
Abstract
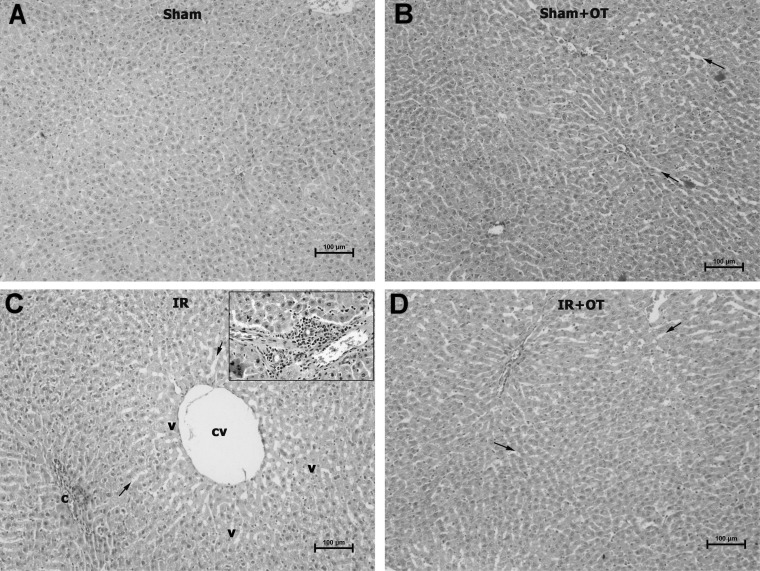
- Renal ischemia-reperfusion (IR) causes remote liver damage. Oxytocin has anti-inflammatory and antioxidant effects. The main purpose of this study was to evaluate the protective function of oxytocin (OT) in remote liver damage triggered by renal IR in rats. Twenty four rats were randomly divided into four different groups, each containing 8 rats. The groups were as follows: (1) Sham operated group; (2) Sham operated+OT group (3) Renal IR group; (4) Renal IR+OT group. OT (500microg/kg) was administered subcutaneously 12 and 24 hours before and immediately after ischemia. At the end of experimental procedure, the rats were sacrificed, and liver specimens were taken for histological assessment or determination of malondialdehyde (MDA), total oxidant status (TOS), total antioxidant status (TAS), paraoxonase (PON-1) activity and nitric oxide (NO). The results showed that renal IR injury constituted a notable elevation in MDA, TOS, Oxidative stress index (OSI) and significantly decreased TAS, PON-1 actvity and NO in liver tissue (p<0.05). Additionally renal IR provoked significant augmentation in hepatic microscopic damage scores. However, alterations in these biochemical and histopathological indices due to IR injury were attenuated by OT treatment (p<0.05). These findings show that OT ameliorates remote liver damage triggered by renal ischemia-reperfusion and this preservation involves suppression of inflammation and regulation of oxidant-antioxidant status.
MeSH Terms
Figure
Reference
-
1. Kadkhodaee M, Golab F, Zahmatkesh M, Ghaznavi R, Hedayati M, Arab HA, Ostad SN, Soleimani M. Effects of different periods of renal ischemia on liver as a remote organ. World J Gastroenterol. 2009; 15:1113–1118. PMID: 19266605.
Article2. Park SW, Chen SW, Kim M, Brown KM, Kolls JK, D'Agati VD, Lee HT. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab Invest. 2011; 91:63–84. PMID: 20697374.
Article3. Serteser M, Koken T, Kahraman A, Yilmaz K, Akbulut G, Dilek ON. Changes in hepatic TNF-alpha levels, antioxidant status, and oxidation products after renal ischemia/reperfusion injury in mice. J Surg Res. 2002; 107:234–240. PMID: 12429181.4. Miyazawa S, Watanabe H, Miyaji C, Hotta O, Abo T. Leukocyte accumulation and changes in extra-renal organs during renal ischemia reperfusion in mice. J Lab Clin Med. 2002; 139:269–278. PMID: 12032487.
Article5. Kaçmaz A, User EY, Sehirli AO, Tilki M, Ozkan S, Sener G. Protective effect of melatonin against ischemia/reperfusion-induced oxidative remote organ injury in the rat. Surg Today. 2005; 35:744–750. PMID: 16133669.
Article6. Petersson M, Wiberg U, Lundeberg T, Uvnäs-Moberg K. Oxytocin decreases carrageenan induced inflammation in rats. Peptides. 2001; 22:1479–1484. PMID: 11514032.
Article7. Barberis C, Mouillac B, Durroux T. Structural bases of vasopressin/oxytocin receptor function. J Endocrinol. 1998; 156:223–229. PMID: 9518866.
Article8. Liberzon I, Young EA. Effects of stress and glucocorticoids on CNS oxytocin receptor binding. Psychoneuroendocrinology. 1997; 22:411–422. PMID: 9364620.
Article9. Petersson M, Lundeberg T, Sohlström A, Wiberg U, Uvnäs-Moberg K. Oxytocin increases the survival of musculocutaneous flaps. Naunyn Schmiedebergs Arch Pharmacol. 1998; 357:701–704. PMID: 9686948.
Article10. Tuğtepe H, Sener G, Biyikli NK, Yüksel M, Cetinel S, Gedik N, Yeğen BC. The protective effect of oxytocin on renal ischemia/reperfusion injury in rats. Regul Pept. 2007; 140:101–108. PMID: 17261335.
Article11. Düşünceli F, Işeri SO, Ercan F, Gedik N, Yeğen C, Yeğen BC. Oxytocin alleviates hepatic ischemia-reperfusion injury in rats. Peptides. 2008; 29:1216–1222. PMID: 18403049.
Article12. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275. PMID: 14907713.
Article13. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358. PMID: 36810.
Article14. Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990; 36:1440–1443. PMID: 2387039.
Article15. Eckerson HW, Wyte CM, La Du BN. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet. 1983; 35:1126–1138. PMID: 6316781.16. Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004; 37:112–119. PMID: 14725941.
Article17. Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005; 38:1103–1111. PMID: 16214125.
Article18. Ozturk E, Balat O, Acılmıs YG, Ozcan C, Pence S, Erel Ö. Measurement of the placental total antioxidant status in preeclamptic women using a novel automated method. J Obstet Gynaecol Res. 2011; 37:337–342. PMID: 21314804.
Article19. Aycicek A, Erel O, Kocyigit A. Increased oxidative stress in infants exposed to passive smoking. Eur J Pediatr. 2005; 164:775–778. PMID: 16025297.
Article20. Star RA. Treatment of acute renal failure. Kidney Int. 1998; 54:1817–1831. PMID: 9853246.
Article21. Gu J, Chen J, Xia P, Tao G, Zhao H, Ma D. Dexmedetomidine attenuates remote lung injury induced by renal ischemia-reperfusion in mice. Acta Anaesthesiol Scand. 2011; 55:1272–1278. PMID: 22092133.
Article22. Awad AS, Kamel R, Sherief MA. Effect of thymoquinone on hepatorenal dysfunction and alteration of CYP3A1 and spermidine/spermine N-1-acetyl-transferase gene expression induced by renal ischaemia-reperfusion in rats. J Pharm Pharmacol. 2011; 63:1037–1042. PMID: 21718287.
Article23. Eschwège P, Paradis V, Conti M, Holstege A, Richet F, Detève J, Ménager P, Legrand A, Jardin A, Bedossa P, Benoit G. In situ detection of lipid peroxidation by-products as markers of renal ischemia injuries in rat kidneys. J Urol. 1999; 162:553–557. PMID: 10411087.24. Işeri SO, Gedik IE, Erzik C, Uslu B, Arbak S, Gedik N, Yeğen BC. Oxytocin ameliorates skin damage and oxidant gastric injury in rats with thermal trauma. Burns. 2008; 34:361–369. PMID: 17826914.
Article25. Vaghasiya JD, Sheth NR, Bhalodia YS, Jivani NP. Exaggerated liver injury induced by renal ischemia reperfusion in diabetes: effect of exenatide. Saudi J Gastroenterol. 2010; 16:174–180. PMID: 20616412.
Article26. Jang HS, Han JH, Jeong JY, Sohn UD. Protective Effect of ECQ on Rat Reflux Esophagitis Model. Korean J Physiol Pharmacol. 2012; 16:455–462. PMID: 23269908.
Article27. Jung J, Nam Y, Sohn UD. Inhibitory Effects of ECQ on Indomethacin-Induced Gastric Damage in Rats. Korean J Physiol Pharmacol. 2012; 16:399–404. PMID: 23269902.
Article28. Davies GR, Simmonds NJ, Stevens TR, Grandison A, Blake DR, Rampton DS. Mucosal reactive oxygen metabolite production in duodenal ulcer disease. Gut. 1992; 33:1467–1472. PMID: 1452069.
Article29. Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004; 37:277–285. PMID: 15003729.
Article30. Cikrikcioglu MA, Hursitoglu M, Erkal H, Kınas BE, Sztajzel J, Cakirca M, Arslan AG, Erek A, Halac G, Tukek T. Oxidative stress and autonomic nervous system functions in restless legs syndrome. Eur J Clin Invest. 2011; 41:734–742. PMID: 21250984.
Article31. Harma M, Harma M, Erel O. Increased oxidative stress in patients with hydatidiform mole. Swiss Med Wkly. 2003; 133:563–566. PMID: 14691728.32. Akkoc H, Kelle I, Tunik S, Bahceci S, Sencar L, Ayaz E, Nergiz Y, Erdinc L, Erdinc M. Protective effect of ethyl pyruvate on liver injury in streptozotocin-induced diabetic rats. Acta Gastroenterol Belg. 2012; 75:336–341. PMID: 23082705.33. Aviram M, Rosenblat M. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med. 2004; 37:1304–1316. PMID: 15454271.
Article34. Yıldırım S, Kısa F, Karadeniz A, Yıldırım A, Karakoc A, Can I, Kara A, Simsek N. Effects of pomegranate seed extract on liver paraoxonase and bcl-xL activities in rats treated with cisplatin. J Med Plant Res. 2012; 6:2317–2323.
Article35. Marsillach J, Bertran N, Camps J, Ferré N, Riu F, Tous M, Coll B, Alonso-Villaverde C, Joven J. The role of circulating monocyte chemoattractant protein-1 as a marker of hepatic inflammation in patients with chronic liver disease. Clin Biochem. 2005; 38:1138–1140. PMID: 16242682.
Article36. Hashemi M, Bahari A, Hashemzehi N, Moazeni-Roodi A, Shafieipour S, Bakhshipour A, Ghavami S. Serum paraoxonase and arylesterase activities in Iranian patients with nonalcoholic fatty liver disease. Pathophysiology. 2012; 19:115–119. PMID: 22555050.
Article37. Siriussawakul A, Zaky A, Lang JD. Role of nitric oxide in hepatic ischemia-reperfusion injury. World J Gastroenterol. 2010; 16:6079–6086. PMID: 21182222.
Article38. Phillips L, Toledo AH, Lopez-Neblina F, Anaya-Prado R, Toledo-Pereyra LH. Nitric oxide mechanism of protection in ischemia and reperfusion injury. J Invest Surg. 2009; 22:46–55. PMID: 19191157.
Article39. Morsy MA. Protective effect of lisinopril on hepatic ischemia/reperfusion injury in rats. Indian J Pharmacol. 2011; 43:652–655. PMID: 22144768.40. Bae EH, Kim SW. Alteration of nitric oxide synthase and guanylyl cyclase activity in rats with ischemia/reperfusion renal injury. Korean J Physiol Pharmacol. 2006; 10:337–341.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes in HO-1, HSP70 and iNOS Expressions in the Rat Liver after Remote Ischemic Preconditioning
- Effects of Remote Ischemic Conditioning Methods on Ischemia-Reperfusion Injury in Muscle Flaps: An Experimental Study in Rats
- The Effect of Allopurinol on the Ischemia-reperfusion Renal Injury in Young Rats
- Ethyl Pyruvate Ameliorates Renal Ischemia- reperfusion Injury
- Pharmacological prevention of ischemia-reperfusion induced liver injury in rats