J Vet Sci.
2011 Dec;12(4):309-317. 10.4142/jvs.2011.12.4.309.
Aerosol delivery of kinase-deficient Akt1 attenuates Clara cell injury induced by naphthalene in the lungs of dual luciferase mice
- Affiliations
-
- 1Laboratory of Toxicology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. mchotox@snu.ac.kr
- 2Department of Nano Fusion Technology, Graduate School of Convergence Science and Technology, Seoul National University, Seoul 151-742, Korea.
- 3Graduate Group of Tumor Biology, Seoul National University, Seoul 151-742, Korea.
- 4Center for Food and Toxicology, Seoul National University, Seoul 151-742, Korea.
- 5Advanced Institute of Convergence Technology, Seoul National University, Suwon 443-270, Korea.
- KMID: 1365013
- DOI: http://doi.org/10.4142/jvs.2011.12.4.309
Abstract
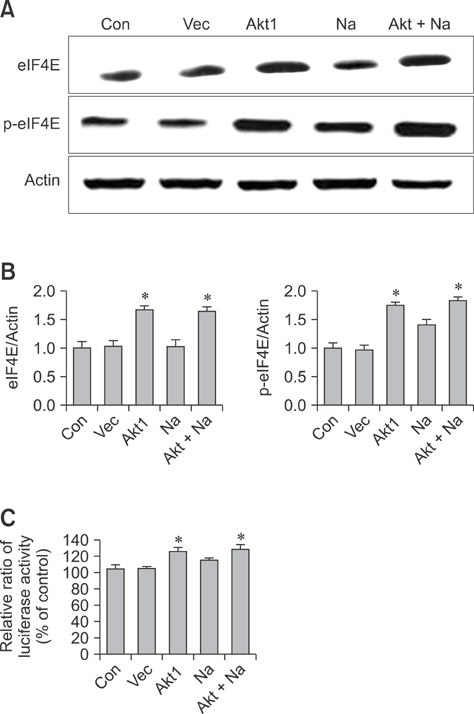
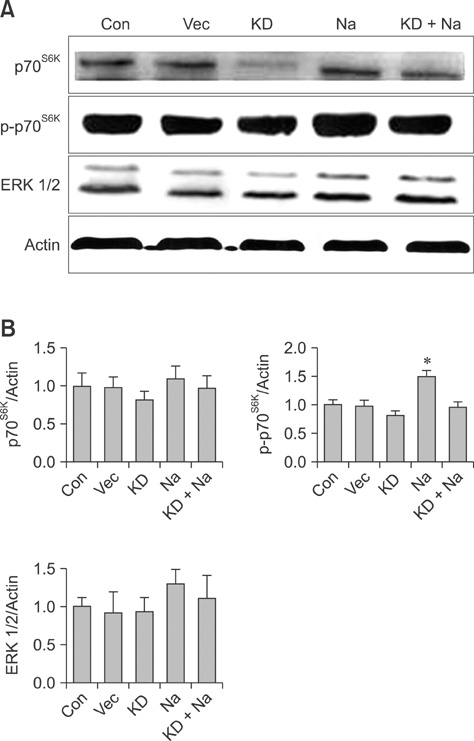
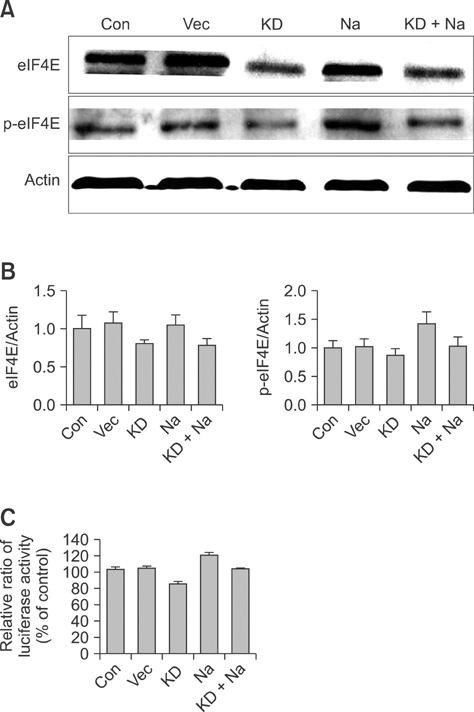
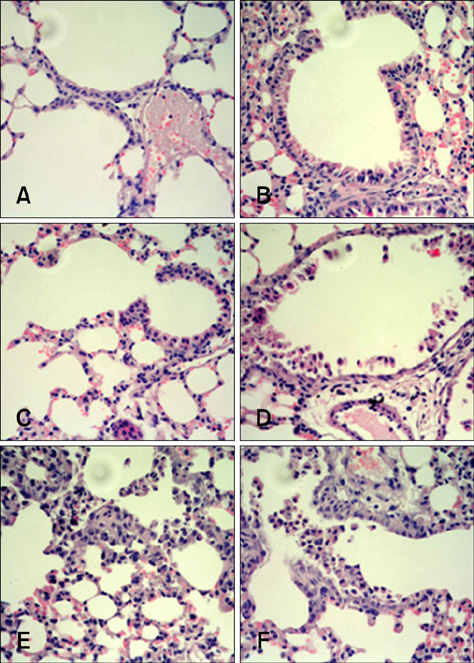
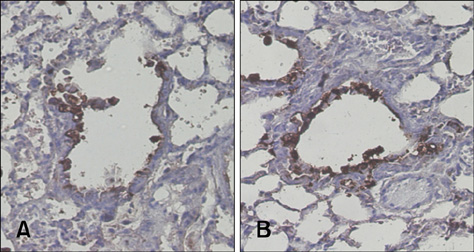
- Conventional lung cancer therapies are associated with poor survival rates; therefore, new approaches such as gene therapy are required for treating cancer. Gene therapies for treating lung cancer patients can involve several approaches. Among these, aerosol gene delivery is a potentially more effective approach. In this study, Akt1 kinase-deficient (KD) and wild-type (WT) Akt1 were delivered to the lungs of CMV-LucR-cMyc-IRES-LucF dual reporter mice through a nose only inhalation system using glucosylated polyethylenimine and naphthalene was administrated to the mice via intraperitoneal injection. Aerosol delivery of Akt1 WT and naphthalene treatment increased protein levels of downstream substrates of Akt signaling pathway while aerosol delivery of Akt1 KD did not. Our results showed that naphthalene affected extracellular signal-regulated kinase (ERK) protein levels, ERK-related signaling, and induced Clara cell injury. However, Clara cell injury induced by naphthalene was considerably attenuated in mice exposed to Akt1 KD. Furthermore, a dual luciferase activity assay showed that aerosol delivery of Akt1 WT and naphthalene treatment enhanced cap-dependent protein translation, while reduced cap-dependent protein translation was observed after delivering Akt1 KD. These studies demonstrated that our aerosol delivery is compatible for in vivo gene delivery.
Keyword
MeSH Terms
-
Administration, Inhalation
Aerosols
Animals
Gene Expression Regulation
Gene Knockdown Techniques
Gene Therapy/*methods
Gene Transfer Techniques
Genes, Reporter
Injections, Intraperitoneal
Luciferases/genetics/*metabolism
Lung Diseases/*chemically induced
Male
Mice
Mice, Transgenic
Naphthalenes/administration & dosage/*toxicity
Proto-Oncogene Proteins c-akt/*administration & dosage/genetics/*metabolism
Figure
Reference
-
1. Avruch J, Belham C, Weng Q, Hara K, Yonezawa K. The p70 S6 kinase integrates nutrient and growth signals to control translational capacity. Prog Mol Subcell Biol. 2001. 26:115–154.
Article2. Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991. 254:274–277.
Article3. Chang RC, Yu MS, Lai CSW. Significance of molecular signaling for protein translation control in neurodegenerative diseases. Neurosignals. 2006-2007. 15:249–258.
Article4. Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene. 2005. 24:7482–7492.
Article5. Commander H. Biotechnology industry responds to gene therapy death. Nat Med. 2000. 6:118.
Article6. Créancier L, Mercier P, Prats AC, Morello D. c-myc Internal ribosome entry site activity is developmentally controlled and subjected to a strong translational repression in adult transgenic mice. Mol Cell Biol. 2001. 21:1833–1840.
Article7. Diodovich C, Malerba I, Bowe G, Acquati F, Bianchi MG, Taramelli R, Parent-Massin D, Gribaldo L. Naphthalene exposure: effects on gene expression and proliferation in human cord blood cells. J Biochem Mol Toxicol. 2003. 17:286–294.
Article8. Dolivet G, Merlin JL, Barberi-Heyob M, Ramacci C, Erbacher P, Parache RM, Behr JP, Guillemin F. In vivo growth inhibitory effect of iterative wild-type p53 gene transfer in human head and neck carcinoma xenografts using glucosylated polyethylenimine nonviral vector. Cancer Gene Ther. 2002. 9:708–714.
Article9. Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997. 275:661–665.
Article10. Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, McConnell R, Kuenzli N, Lurmann F, Rappaport E, Margolis H, Bates D, Peters J. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004. 351:1057–1067.
Article11. Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999. 68:913–963.
Article12. Godbey WT, Wu KK, Hirasaki GJ, Mikos AG. Improved packing of poly(ethylenimine)/DNA complexes increases transfection efficiency. Gene Ther. 1999. 6:1380–1388.
Article13. Goula D, Becker N, Lemkine GF, Normandie P, Rodrigues J, Mantero S, Levi G, Demeneix BA. Rapid crossing of the pulmonary endothelial barrier by polyethylenimine/DNA complexes. Gene Ther. 2000. 7:499–504.
Article14. Griesenbach U, Chonn A, Cassady R, Hannam V, Ackerley C, Post M, Tanswell AK, Olek K, O'Brodovich H, Tsui L-C. Comparison between intratracheal and intravenous administration of liposome-DNA complexes for cystic fibrosis lung gene therapy. Gene Ther. 1998. 5:181–188.
Article15. Hwang SK, Jin H, Kwon JT, Chang SH, Kim TH, Cho CS, Lee KH, Young MR, Colburn NH, Beck GR Jr, Yang HS, Cho MH. Aerosol-delivered programmed cell death 4 enhanced apoptosis, controlled cell cycle and suppressed AP-1 activity in the lungs of AP-1 luciferase reporter mice. Gene Ther. 2007. 14:1353–1361.
Article16. Javle MM, Gibbs JF, Iwata KK, Pak Y, Rutledge P, Yu J, Black JD, Tan D, Khoury T. Epithelial-mesenchymal transition (EMT) and activated extracellular signal-regulated kinase (p-Erk) in surgically resected pancreatic cancer. Ann Surg Oncol. 2007. 14:3527–3533.
Article17. Jefferies HBJ, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5'-TOP mRNA translation through inhibition of p70S6k. EMBO J. 1997. 16:3693–3704.
Article18. Kim HW, Park IK, Cho CS, Lee KH, Beck GR Jr, Colburn NH, Cho MH. Aerosol delivery of glucosylated polyethylenimine/phosphatase and tensin homologue deleted on chromosome 10 complex suppresses Akt downstream pathways in the lung of K-ras null mice. Cancer Res. 2004. 64:7971–7976.
Article19. Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature. 1990. 345:544–547.
Article20. Lee Y, Cho MY, Mo H, Nam K, Koo H, Jin G, Park JS. Enhancement of the transfection efficiency of poly (ethylenimine) by guanidylation. Bull Korean Chem Soc. 2008. 29:666–668.21. Leikauf GD. Hazardous air pollutants and asthma. Environ Health Perspect. 2002. 110:Suppl 4. 505–526.
Article22. Li J, Simpson L, Takahashi M, Miliaresis C, Myers MP, Tonks N, Parsons R. The PTEN/MMAC1 tumor suppressor induces cell death that is rescued by the AKT/protein kinase B oncogene. Cancer Res. 1998. 58:5667–5672.23. Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003. 4:257–262.24. Massion PP, Taflan PM, Shyr Y, Rahman SM, Yildiz P, Shakthour B, Edgerton ME, Ninan M, Andersen JJ, Gonzalez AL. Early involvement of the phosphatidylinositol 3-kinase/Akt pathway in lung cancer progression. Am J Respir Crit Care Med. 2004. 170:1088–1094.
Article25. Merdan T, Kopeček J, Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv Drug Deliv Rev. 2002. 54:715–758.
Article26. Merlin JL, Dolivet G, Dubessy C, Festor E, Parache RM, Verneuil L, Erbacher P, Behr JP, Guillemin F. Improvement of nonviral p53 gene transfer in human carcinoma cells using glucosylated polyethylenimine derivatives. Cancer Gene Ther. 2001. 8:203–210.
Article27. Mitsiades CS, Mitsiades N, Koutsilieris M. The Akt pathway: molecular targets for anti-cancer drug development. Curr Cancer Drug Targets. 2004. 4:235–256.
Article28. Monick MM, Powers LS, Barrett CW, Hinde S, Ashare A, Groskreutz DJ, Nyunoya T, Coleman M, Spitz DR, Hunninghake GW. Constitutive ERK MAPK activity regulates macrophage ATP production and mitochondrial integrity. J Immunol. 2008. 180:7485–7496.
Article29. Park IK, Cook SE, Kim YK, Kim HW, Cho MH, Jeong HJ, Kim EM, Nah JW, Bom HS, Cho CS. Glucosylated polyethylenimine as a tumor-targeting gene carrier. Arch Pharm Res. 2005. 28:1302–1310.
Article30. Pfeifer A, Verma IM. Gene therapy: promises and problems. Annu Rev Genomics Hum Genet. 2001. 2:177–211.
Article31. Plopper CG, Macklin J, Nishio SJ, Hyde DM, Buckpitt AR. Relationship of cytochrome P-450 activity to Clara cell cytotoxicity. III. Morphometric comparison of changes in the epithelial populations of terminal bronchioles and lobar bronchi in mice, hamsters, and rats after parenteral administration of naphthalene. Lab Invest. 1992. 67:553–565.32. Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006. 3:726–733.
Article33. Qian J, Zou Y, Rahman JSM, Lu B, Massion PP. Synergy between phosphatidylinositol 3-kinase/Akt pathway and Bcl-xL in the control of apoptosis in adenocarcinoma cells of the lung. Mol Cancer Ther. 2009. 8:101–109.
Article34. Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J. RAS/ERK Signaling Promotes Site-specific Ribosomal Protein S6 Phosphorylation via RSK and Stimulates Cap-dependent Translation. J Biol Chem. 2007. 282:14056–14064.
Article35. Ruggero D, Montanaro L, Ma L, Xu W, Londei P, Cordon-Cardo C, Pandolfi PP. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med. 2004. 10:484–486.
Article36. Ruggero D, Pandolfi PP. Does the ribosome translate cancer? Nat Rev Cancer. 2003. 3:179–192.
Article37. Ruggero D, Sonenberg N. The Akt of translational control. Oncogene. 2005. 24:7426–7434.
Article38. Scheper GC, Proud CG. Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur J Biochem. 2002. 269:5350–5359.
Article39. Shah A, Swain WA, Richardson D, Edwards J, Stewart DJ, Richardson CM, Swinson DEB, Patel D, Jones JL, O'Byrne KJ. Phospho-Akt expression is associated with a favorable outcome in non-small cell lung cancer. Clin Cancer Res. 2005. 11:2930–2936.
Article40. Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA. 1987. 84:5034–5037.
Article41. Tehrani AM, Hwang SK, Kim TH, Cho CS, Hua J, Nah WS, Kwon JT, Kim JS, Chang SH, Yu KN, Park SJ, Bhandari DR, Lee KH, An GH, Beck GR Jr, Cho MH. Aerosol delivery of Akt controls protein translation in the lungs of dual luciferase reporter mice. Gene Ther. 2007. 14:451–458.
Article42. Templeton NS, Lasic DD. New directions in liposome gene delivery. Mol Biotechnol. 1999. 11:175–180.
Article43. Uduehi AN, Stammberger U, Kubisa B, Gugger M, Buehler TA, Schmid RA. Effects of linear polyethylenimine and polyethylenimine/DNA on lung function after airway instillation to rat lungs. Mol Ther. 2001. 4:52–57.
Article44. Wang L, Proud CG. Ras/Erk signaling is essential for activation of protein synthesis by Gq protein-coupled receptor agonists in adult cardiomyocytes. Circ Res. 2002. 91:821–829.
Article45. Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997. 16:1909–1920.
Article46. Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004. 428:332–337.
Article47. Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Teruya-Feldstein J, Pelletier J, Lowe SW. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007. 21:3232–3237.
Article48. West JA, Buckpitt AR, Plopper CG. Elevated airway GSH resynthesis confers protection to Clara cells from naphthalene injury in mice made tolerant by repeated exposures. J Pharmacol Exp Ther. 2000. 294:516–523.49. West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003. 111:81–90.
Article50. Witschi H, Espiritu I, Maronpot RR, Pinkerton KE, Jones AD. The carcinogenic potential of the gas phase of environmental tobacco smoke. Carcinogenesis. 1997. 18:2035–2042.
Article51. Yildirim AÖ, Veith M, Rausch T, Müller B, Kilb P, Van Winkle LS, Fehrenbach H. Keratinocyte growth factor protects against Clara cell injury induced by naphthalene. Eur Respir J. 2008. 32:694–704.
Article52. Zaric V, Weltin D, Erbacher P, Remy JS, Behr JP, Stephan D. Effective polyethylenimine-mediated gene transfer into human endothelial cells. J Gene Med. 2004. 6:176–184.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Akt1 in renal fibrosis and tubular dedifferentiation during the progression of acute kidney injury to chronic kidney disease
- Role for CD40 and CD40L Expression in Generating CD8 T Cell Response to Minor Histcompatibility Antigen, H60
- Lysophosphatidic acid induces cell migration through the selective activation of Akt1
- Ginsenoside Rg3 attenuates ischemia reperfusion injury via adenosine monophosphate-activated protein kinase-mediated autophagy flux
- Deficiency of Sphingosine-1-Phosphate Receptor 2 (S1Pâ‚‚) Attenuates Bleomycin-Induced Pulmonary Fibrosis