J Vet Sci.
2006 Mar;7(1):13-17. 10.4142/jvs.2006.7.1.13.
Increased phosphorylation of c-Jun NH (2)-terminal protein kinase in the sciatic nerves of Lewis rats with experimental autoimmune neuritis
- Affiliations
-
- 1Department of Veterinary Medicine, Cheju National University, Jeju 690-756, Korea. shint@cheju.ac.kr
- KMID: 1103547
- DOI: http://doi.org/10.4142/jvs.2006.7.1.13
Abstract
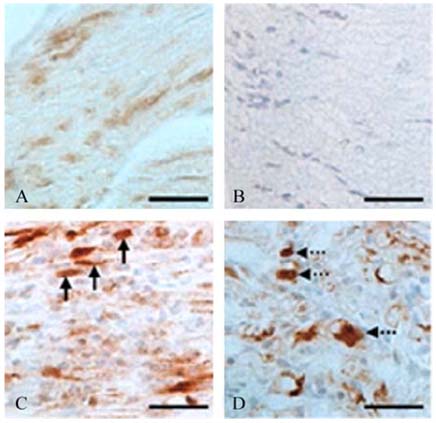
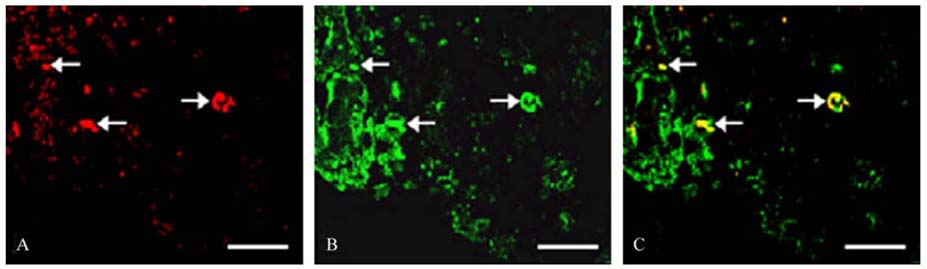
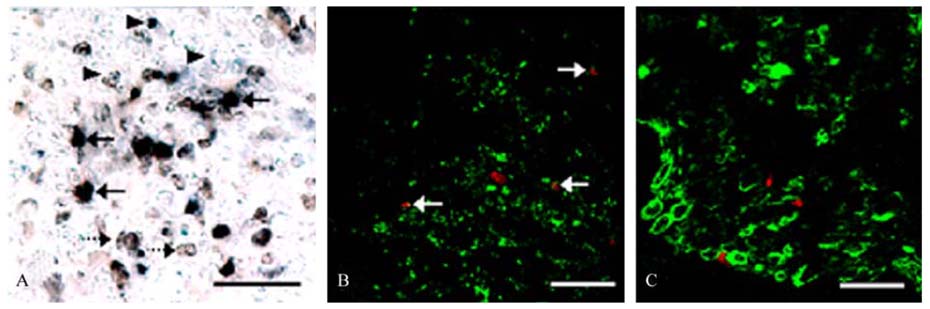
- The phosphorylation of c-Jun NH (2)-terminal protein kinase (JNK), one of the mitogen-activated protein kinases, was analyzed in the sciatic nerves of Lewis rats with experimental autoimmune neuritis (EAN). Western blot analysis showed that the expression levels of both phosphorylated JNK1 (p-JNK1, approximately 46 kDa) and phosphorylated JNK2 (p-JNK2, approximately 54 kDa) in the sciatic nerves of rats with EAN increased significantly (p < 0.05) at day 14 post-immunization (PI) and remained at this level at days 24 and 30 PI, with a slight decrease. In EANaffected sciatic nerves, there was intense immunostaining for p-JNK in the infiltrating inflammatory cells (especially ED1- positive macrophages) and Schwann cells on days 14-24 PI, compared with those of controls. Some macrophages with increased p-JNK immunoreactivity was shown to be apoptotic, while some Schwann cells remained survived in this rat EAN model, suggesting that JNK is differentially involved in the EAN-affected sciatic nerves. These findings suggest that JNK phosphorylation is closely associated with the clearance of inflammatory cells as well as the activation of Schwann cells in the EAN affected sciatic nerves.
Keyword
MeSH Terms
-
Animals
Apoptosis/physiology
Blotting, Western
Ectodysplasins
Female
Immunohistochemistry
In Situ Nick-End Labeling
JNK Mitogen-Activated Protein Kinases/*metabolism
Membrane Proteins/metabolism
Neuritis, Autoimmune, Experimental/*enzymology/metabolism/pathology
Phosphorylation
Rats
Rats, Inbred Lew
S100 Proteins/metabolism
Schwann Cells/metabolism
Sciatic Nerve/*enzymology/metabolism/pathology
Tumor Necrosis Factors/metabolism
Figure
Reference
-
1. Ahn M, Moon C, Lee Y, Koh CS, Kohyama K, Tanuma N, Matsumoto Y, Kim HM, Kim SR, Shin T. Activation of extracellular signal-regulated kinases in the sciatic nerves of rats with experimental autoimmune neuritis. Neurosci Lett. 2004. 372:57–61.
Article2. Chang L, Karin MK. Mammalian MAP kinase signaling cascades. Nature. 2001. 410:37–40.3. Cheng HL, Steinway ML, Xin X, Feldman EL. Insulin-like growth factor-I and Bcl-X (L) inhibit c-jun N-terminal kinase activation and rescue Schwann cells from apoptosis. J Neurochem. 2001. 76:935–943.
Article4. Conti G, Scarpini E, Rostami A, Livraghi S, Baron PL, Pleasure D, Scarlato G. Schwann cell undergoes apoptosis during experimental allergic neuritis (EAN). J Neurol Sci. 1998. 161:29–35.
Article5. Gold R, Hartung HP, Toyka KV. Animal models for autoimmune demyelinating disorders of the nervous system. Mol Med Today. 2000. 6:88–91.
Article6. Hartung HP, Toyka KV. T-cell and macrophage activation in experimentally induced autoimmune neuritis and Guillain-Barre syndrome. Ann Neurol. 1990. 27:S57–S63.7. Lee Y, Shin T. Expression of constitutive endothelial and inducible nitric oxide synthase in the sciatic nerve of Lewis rats with experimental autoimmune neuritis. J Neuroimmunol. 2002. 126:78–85.
Article8. Moon C, Ahn M, Kim H, Lee Y, Koh CS, Matsumoto Y, Shin T. Activation of p38 mitogen-activated protein kinase in the early and peak phases of autoimmune neuritis in rat sciatic nerves. Brain Res. 2005. 1040:208–213.
Article9. Moon C, Kim S, Wie M, Kim H, Cheong J, Park J, Jee Y, Tanuma N, Matsumoto Y, Shin T. Increased expression of p53 and Bax in the spinal cords of rats with experimental autoimmune encephalomyelitis. Neurosci Lett. 2000. 289:41–44.
Article10. Nishina H, Nakagawa K, Azuma N, Katada T. Activation mechanism and physiological roles of stress-activated protein kinase/c-Jun NH2-terminal kinase in mammalian cells. J Biol Regul Homeost Agents. 2003. 17:295–302.11. Nishina H, Wada T, Katada T. Physiological roles of SAPK/JNK signaling pathway. J Biochem. 2004. 136:123–126.
Article12. Shin T, Ahn M, Jung K, Heo S, Kim D, Jee Y, Lim YK, Yeo EJ. Activation of mitogen-activated protein kinases in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2003. 140:118–125.
Article13. Shin T, Min DS, Ahn M, Son W, Matsumoto Y. Increased expression of phospholipase D1 in the sciatic nerve of rats with experimental autoimmune neuritis. Immunol Invest. 2002. 31:169–176.
Article14. Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995. 270:1326–1331.
Article15. Zhu J, Mix E, Link H. Cytokine production and the pathogenesis of experimentally induced autoimmune neuritis and Guillain-Barre syndrome. J Neuroimmunol. 1998. 84:40–52.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lithium alleviates paralysis in experimental autoimmune neuritis in Lewis rats by modulating glycogen synthase kinase-3β activity
- Increased expression of osteopontin in the spinal cords of Lewis rats with experimental autoimmune neuritis
- Mechanism of experimental autoimmune encephalomyelitis in Lewis rats: recent insights from macrophages
- Multiple Regulation of Roundabout (Robo) Phosphorylation in a Heterologous Cell System
- Involvement of JNK-initiated p53 accumulation and phosphorylation of p53 in pseudolaric acid B induced cell death