Korean J Ophthalmol.
2010 Apr;24(2):101-107. 10.3341/kjo.2010.24.2.101.
Protective Effect of Catechin on Apoptosis of the Lens Epithelium in Rats with N-methyl-N-nitrosourea-induced Cataracts
- Affiliations
-
- 1Department of Ophthalmology, Myongji Hospital, Kwandong University College of Medicine, Goyang, Korea. bnkang@kd.ac.kr
- 2Department of physiology, Kyung-Hee University College of Medicine, Seoul, Korea.
- 3Department of Ophthalmology, Chungju Hospital, Konkuk University School of Medicine, Chungju, Korea.
- KMID: 946002
- DOI: http://doi.org/10.3341/kjo.2010.24.2.101
Abstract
- PURPOSE
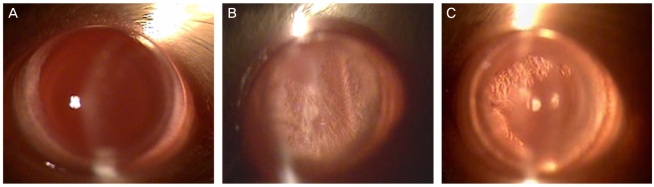
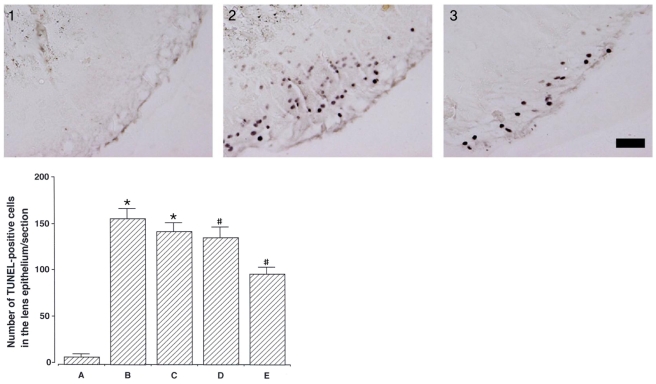
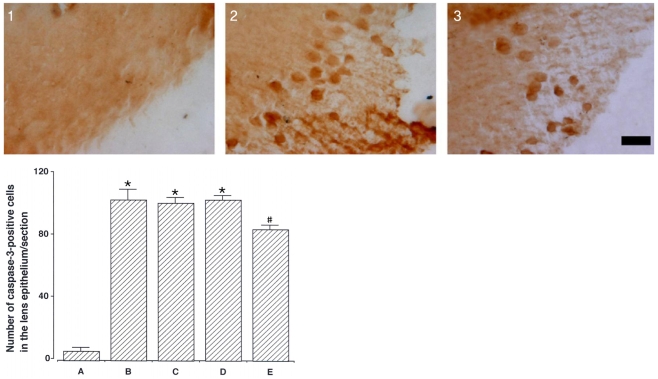
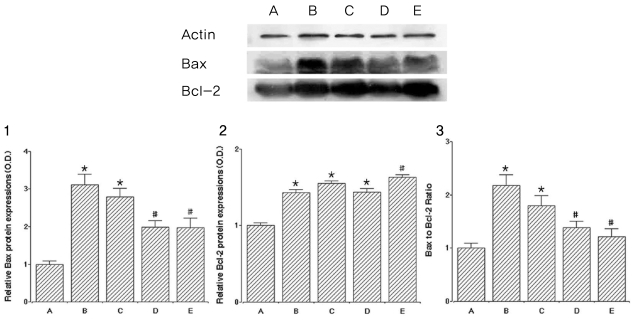
To investigate the effect of catechin on apoptotic cell death in the lens epithelium of rats with cataract. METHODS: Cataract was induced by intraperitoneal injection of 100 mg/kg N-methyl-N-nitrosourea (MNU) to ten day-old Sprague-Dawley rats. The neonatal rats were randomly divided into five groups (n=15 in each group): a control group, and four cataract-induction groups, treated with either 0, 50, 100, 200 mg/kg catechin. We performed slit-lamp biomicroscopic analysis, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay, Western-blot for Bcl-2 and Bax, and immunohistochemistry for caspase-3. RESULTS: Apoptotic cell death in lens epithelial cells that increased following cataract formation in rats was suppressed by cathechin. CONCLUSIONS: Catechin inhibited cataract-induced apoptotic cell death in the lens epithelium and may prove useful for the prevention of cataract progression.
Keyword
MeSH Terms
Figure
Reference
-
1. Harocopos GJ, Alvares KM, Kolker AE, Beebe DC. Human age-related cataract and lens epithelial cell death. Invest Ophthalmol Vis Sci. 1998; 39:2696–2706. PMID: 9856780.2. Bassnett S, Kuszak JR, Reinisch L, et al. Intercellular communication between epithelial and fiber cells of the eye lens. J Cell Sci. 1994; 107:799–811. PMID: 8056837.
Article3. Hightower KR, Reddan JR, McCready JP, Dziedzic DC. Lens epithelium: a primary target of UVB irradiation. Exp Eye Res. 1994; 59:557–564. PMID: 9492757.
Article4. Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide ranging implications in tissue kinetics. Br J Cancer. 1972; 26:239–257. PMID: 4561027.5. Woodle ES, Kulkarni S. Programmed cell death. Transplantation. 1998; 66:681–691. PMID: 9771830.
Article6. Lolley RN. The rd gene defect triggers programmed rod cell death. The proctor lecture. Invest Ophthalmol Vis Sci. 1994; 35:4182–4191. PMID: 8002239.7. Papermaster DS, Windle J. Death at an early age. Apoptosis in inherited retina degenerations. Invest Ophthalmol Vis Sci. 1995; 36:977–983. PMID: 7730031.8. Li WC. The lens epithelium, apoptosis and cataract formation. Nova Acta Leopoldina. 1997; 75:81–108.9. Li WC, Kuszak JR, Dunn K, et al. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J Cell Biol. 1995; 130:169–181. PMID: 7790371.
Article10. Li WC, Spector A. Lens epithelial cell apoptosis is an early event in the development of UVB-induced cataract. Free Radic Biol Med. 1996; 20:301–311. PMID: 8720900.
Article11. Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992; 119:493–501. PMID: 1400587.
Article12. Vaux DL, Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci USA. 1996; 93:2239–2244. PMID: 8637856.
Article13. Charakidas A, Kalogeraki A, Tsilimbaris M, et al. Lens epithelial apoptosis and cell proliferation in human age-related cortical cataract. Eur J Ophthalmol. 2005; 15:213–220. PMID: 15812762.
Article14. Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997; 326:1–16. PMID: 9337844.
Article15. Upadhyay D, Panduri V, Ghio A, Kamp DW. Particulate matter induces alveolar epithelial cell DNA damage and apoptosis: role of free radicals and the mitochondria. Am J Respir Cell Mol Biol. 2003; 29:180–187. PMID: 12600817.16. Bun SS, Bun H, Guédon D, et al. Effect of green tea extracts on liver functions in Wistar rats. Food Chem Toxicol. 2006; 44:1108–1113. PMID: 16487645.
Article17. Nakagawa T, Yokozawa T. Direct scavenging of nitric oxide and superoxide by green tea. Food Chem Toxicol. 2002; 40:1745–1750. PMID: 12419687.
Article18. Nagarajan S, Nagarajan R, Braunhut SJ, et al. Biocatalytically oligomerized epicatechin with potent and specific anti-proliferative activity for human breast cancer cells. Molecules. 2008; 13:2704–2716. PMID: 18978700.
Article19. Lim YC, Lee SH, Song MH, et al. Growth inhibition and apoptosis by (-)-epicatechin gallate are mediated by cyclin D1 suppression in head and neck squamous carcinoma cells. Eur J Cancer. 2006; 42:3260–3266. PMID: 17045795.
Article20. Berletch JB, Liu C, Love WK, et al. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J Cell Biochem. 2008; 103:509–519. PMID: 17570133.
Article21. Yang SW, Lee BR, Koh JW. Protective effects of epigallocatechin gallate after UV irradiation in cultured human retinal pigment epithelial cells. Korean J Ophthalmol. 2007; 21:232–237. PMID: 18063889.
Article22. Heo J, Lee BR, Koh JW. Protective effects of epigallocatechin gallate after UV irradiation of cultured human lens epithelial cells. Korean J Ophthalmol. 2007; 22:183–186. PMID: 18784447.
Article23. Siegers CP, Michael V, Gisela S, Younes M. Effects of dithioearb and (+)-eateehin against carbon tetrachloride-alcohol-induced liver fibrosis. Agents and Actions. 1982; 12:743–748. PMID: 6299080.24. Anjaneyulu M, Tirkey N, Chopra K. Attenuation of cyclosporine-induced renal dysfunction by catechin: possible antioxidant mechanism. Ren Fail. 2003; 25:691–707. PMID: 14575278.
Article25. Kiuchi K, Yoshizawa K, Moriguchi K, Tsubura A. Rapid induction of cataract by a single intraperitoneal administration of N-methyl-N-nitrosourea in 15-day-old Sprague-Dawley (Jcl: SD) rats. Exp Toxicol Pathol. 2002; 54:181–186. PMID: 12484553.26. Murata M, Ohta N, Sakurai S, et al. The role of aldose reductase in sugar cataract formation: aldose reductase plays a key role in lens epithelial cell death (apoptosis). Chem Biol Interact. 2001; 130-132:617–625. PMID: 11306080.
Article27. Sim YJ, Kim SS, Kim JY, et al. Treadmill exercise improves short-term memory by suppressing ischemia-induced apoptosis of neuronal cells in gerbils. Neurosci Lett. 2004; 372:256–261. PMID: 15542251.
Article28. Gelatt KN, Das ND. Animal models for inherited cataracts: a review. Curr Eye Res. 1984; 3:765–778. PMID: 6734258.
Article29. Roy B, Fujimoto N, Watanabe H, Ito A. Induction of cataract in methylnitrosourea treated Fischer (F344) rats. Hiroshima J Med Sci. 1989; 38:95–98. PMID: 2584061.30. Yoshizawa K, Oishi Y, Nambu H, et al. Cataractogenesis in neonatal Sprague-Dawley rats by N-methyl-N-nitrosourea. Toxicol Pathol. 2000; 28:555–564. PMID: 10930042.31. Lee EH, Wan XH, Song J, et al. Lens epithelial cell death and reduction of anti-apoptotic protein Bcl-2 in human anterior polar cataracts. Mol Vis. 2002; 8:235–240. PMID: 12118239.32. Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995; 9:1173–1182. PMID: 7672510.
Article33. Li WC, Kuszak JR, Wang GM, et al. Calcimycin-induced lens epithelial cell apoptosis contributes to cataract formation. Exp Eye Res. 1995; 61:91–98. PMID: 7556474.
Article34. Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996; 88:386–401. PMID: 8695785.
Article35. Akhtar RS, Ness JM, Roth KA. Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim Biophys Acta. 2004; 1644:189–203. PMID: 14996503.
Article36. Reed JC, Jurgensmeier JM, Matsuyama S. Bcl-2 family proteins and mitochondria. Biochim Biophys Acta. 1998; 10:127–137. PMID: 9714773.
Article37. Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003; 15:691–699. PMID: 14644193.
Article38. Roy AM, Baliga MS, Katiyar SK. Epigallocatechin-3-gallate induces apoptosis in estrogen receptor-negative human breast carcinoma cells via modulation in protein expression of p53 and Bax and caspase-3 activation. Mol Cancer Ther. 2005; 4:81–90. PMID: 15657356.39. Nishikawa T, Nakajima T, Moriguchi M, et al. A green tea polyphenol, epigalocatechin-3-gallate, induces apoptosis of human hepatocellular carcinoma, possibly through inhibition of Bcl-2 family proteins. J Hepatol. 2006; 44:1074–1082. PMID: 16481065.
Article40. Noda C, He J, Takano T, et al. Induction of apoptosis by epigallocatechin-3-gallate in human lymphoblastoid B cells. Biochem Biophys Res Commun. 2007; 362:951–957. PMID: 17803956.
Article41. Tang Y, Zhao DY, Elliott S, et al. Epigallocatechin-3 gallate induces growth inhibition and apoptosis in human breast cancer cells through survivin suppression. Int J Oncol. 2007; 31:705–711. PMID: 17786300.
Article42. Yao K, Ye PP, Zhang L, et al. Epigallocatechin gallate protects against oxidative stress-induced mitochondria-dependent apoptosis in human lens epithelial cells. Mol Vis. 2008; 14:217–223. PMID: 18334937.43. Levites Y, Amit T, Youdim MB, Mandel S. Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (-)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem. 2002; 277:30574–30580. PMID: 12058035.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Esculetin, a Coumarin Derivative, Inhibits Aldose Reductase Activity in vitro and Cataractogenesis in Galactose-Fed Rats
- The Effect of Verapamil on Calcium Transport in the Lens of the Diabetic Rat
- Morphological and functional evaluation of an animal model for the retinal degeneration induced by N-methyl-N-nitrosourea
- The Antihyperplastic Effect of Oral Catechin Ingestion in a Rat Model of Benign Prostatic Hyperplasia
- The Effect of Green Tea Catechin on Lymphocytic Leukemia Cell Line