Anat Cell Biol.
2011 Dec;44(4):314-323. 10.5115/acb.2011.44.4.314.
Morphological and functional evaluation of an animal model for the retinal degeneration induced by N-methyl-N-nitrosourea
- Affiliations
-
- 1Department of Anatomy, Catholic Institute for Advanced Biomaterials, The Catholic University of Korea College of Medicine, Seoul, Korea. ibkimmd@catholic.ac.kr
- KMID: 1447445
- DOI: http://doi.org/10.5115/acb.2011.44.4.314
Abstract
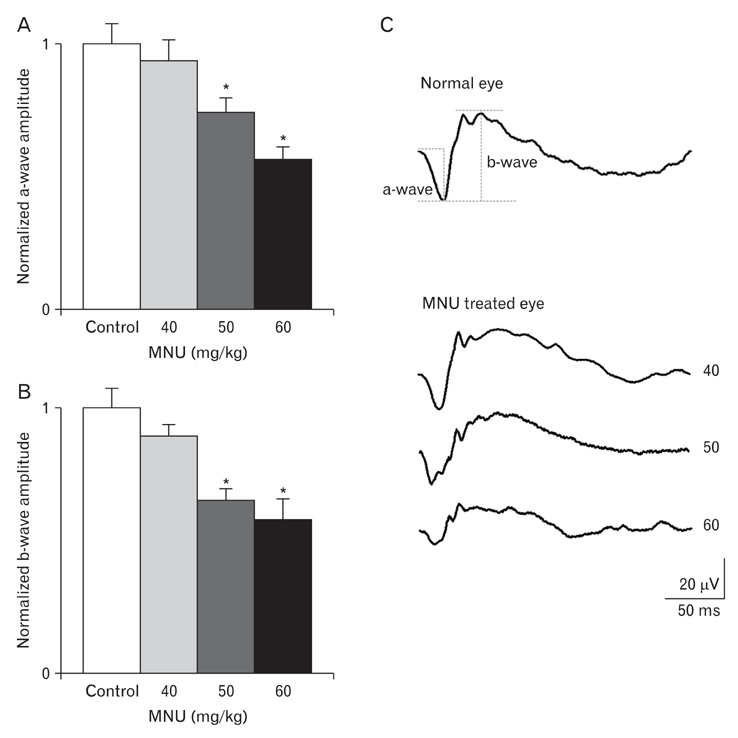
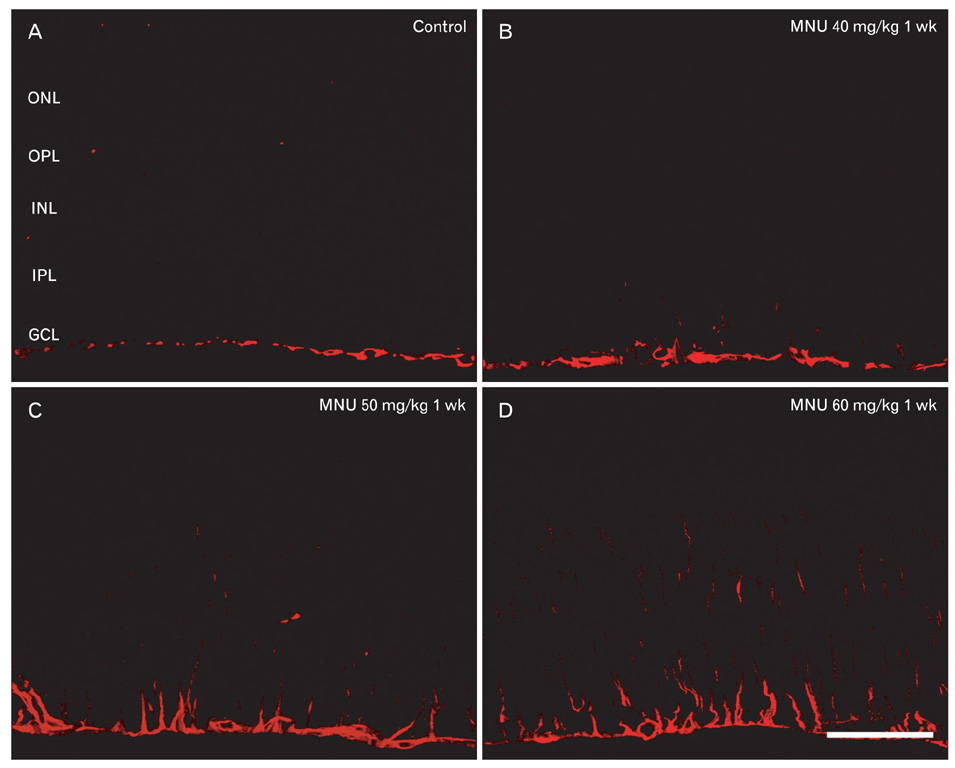
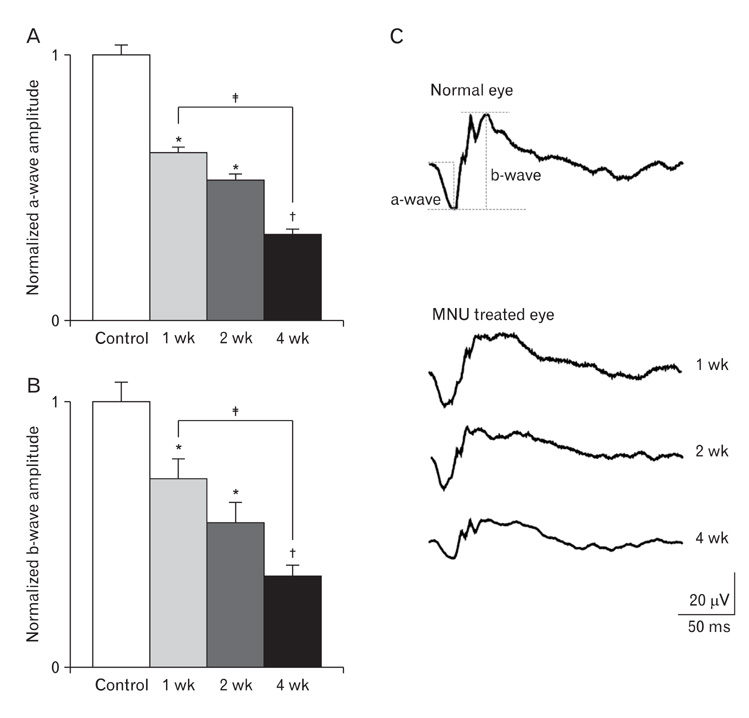
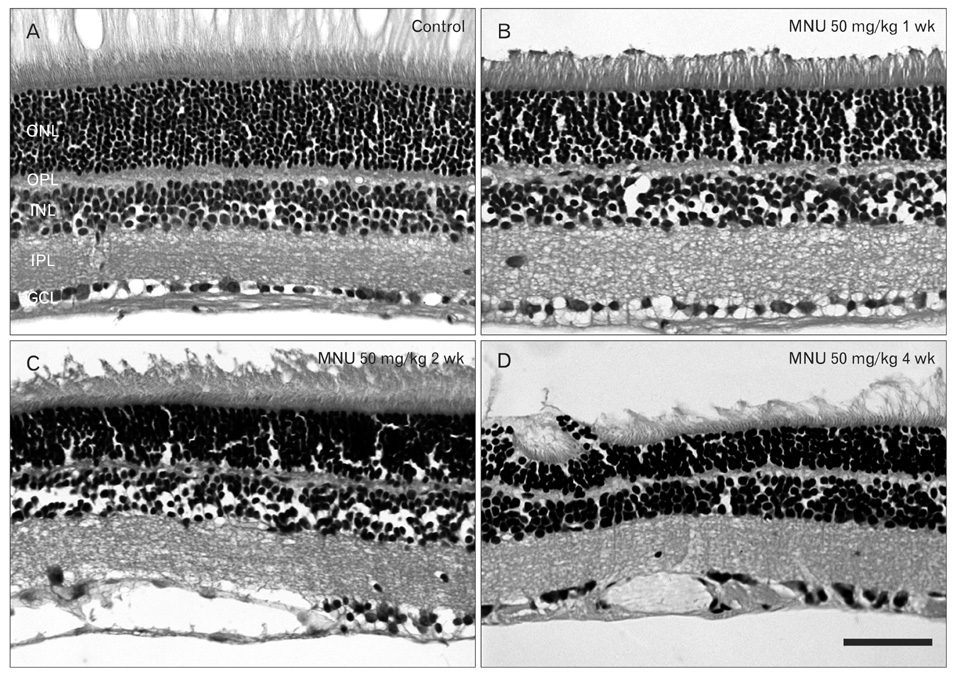
- The retinal degeneration (RD) is a general cause of blindness. To study its pathophysiology and evaluate the effects of new therapeutic agents before clinical trials, it is essential to establish reliable and stable animal models. This study evaluated a RD animal model in which blindness was induced by N-methyl-N-nitrosourea (MNU), a potent retinotoxin leading to apoptosis of photoreceptors. MNU was applied to the Sprague-Dawley rats by a single intraperitoneal injection in different doses (40, 50, and 60 mg/kg). The retinal functions were examined at 1 week after MNU injection by electroretinogram (ERG). Afterwards, each retina was examined by hematoxylin and eosin stain and immunohistochemistry with anti-glial fibrillary acidic protein antibody. Upon MNU injection of 40, 50 and 60 mg/kg, the ERG amplitude of a-waves showed significant reductions of 7, 26, and 44%, respectively, when compared to that of normal a-waves. The b-wave amplitudes were about 89, 65, and 58% of normal b-waves in the response to scotopic light stimulus. At 1 week, 2 weeks, and 4 weeks after MNU injection (50 mg/kg), all scotopic ERG components decreased progressively. In addition, degeneration of retinal neurons was observed in a time- and dose-dependent manner after MNU injection. Taken together, functional reduction following RD induced by MNU correlates with morphological changes. Thus, this RD rat model may be a useful model to study its pathophysiology and to evaluate the effects of new therapeutic agents before clinical trials.
MeSH Terms
Figure
Reference
-
1. Chopdar A, Chakravarthy U, Verma D. Age related macular degeneration. BMJ. 2003. 326:485–488.2. Gregory-Evans K, Bhattacharya SS. Genetic blindness: current concepts in the pathogenesis of human outer retinal dystrophies. Trends Genet. 1998. 14:103–108.3. Papermaster DS, Windle J. Death at an early age. Apoptosis in inherited retinal degenerations. Invest Ophthalmol Vis Sci. 1995. 36:977–983.4. Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990. 347:677–680.5. Sanyal S, De Ruiter A, Hawkins RK. Development and degeneration of retina in rds mutant mice: light microscopy. J Comp Neurol. 1980. 194:193–207.6. Zack DJ. Birth and death in the retina: more related than we thought? Neuron. 1999. 23:411–412.7. Heywood R, Gopinath C. Morphological assessment of visual dysfunction. Toxicol Pathol. 1990. 18(1 Pt 2):204–217.8. Herrold KM. Pigmentary degeneration of the retina induced by N-methyl-N-nitrosourea. An experimental study in syrian hamsters. Arch Ophthalmol. 1967. 78:650–653.9. Nakajima M, Yuge K, Senzaki H, Shikata N, Miki H, Uyama M, Tsubura A. Photoreceptor apoptosis induced by a single systemic administration of N-methyl-N-nitrosourea in the rat retina. Am J Pathol. 1996. 148:631–641.10. Nambu H, Yuge K, Nakajima M, Shikata N, Takahashi K, Miki H, Uyama M, Tsubura A. Morphologic characteristics of N-methyl-N-nitrosourea-induced retinal degeneration in C57BL mice. Pathol Int. 1997. 47:377–383.11. Ogino H, Ito M, Matsumoto K, Yagyu S, Tsuda H, Hirono I, Wild CP, Montesano R. Retinal degeneration induced by N-methyl-N-nitrosourea and detection of 7-methyldeoxyguanosine in the rat retina. Toxicol Pathol. 1993. 21:21–25.12. Oka T, Nakajima T, Tamada Y, Shearer TR, Azuma M. Contribution of calpains to photoreceptor cell death in N-methyl-Nnitrosourea-treated rats. Exp Neurol. 2007. 204:39–48.13. Petrin D, Baker A, Coupland SG, Liston P, Narang M, Damji K, Leonard B, Chiodo VA, Timmers A, Hauswirth W, Korneluk RG, Tsilfidis C. Structural and functional protection of photoreceptors from MNU-induced retinal degeneration by the X-linked inhibitor of apoptosis. Invest Ophthalmol Vis Sci. 2003. 44:2757–2763.14. Taomoto M, Nambu H, Senzaki H, Shikata N, Oishi Y, Fujii T, Miki H, Uyama M, Tsubura A. Retinal degeneration induced by N-methyl-N-nitrosourea in Syrian golden hamsters. Graefes Arch Clin Exp Ophthalmol. 1998. 236:688–695.15. Yuge K, Nambu H, Senzaki H, Nakao I, Miki H, Uyama M, Tsubura A. N-methyl-N-nitrosourea-induced photoreceptor apoptosis in the mouse retina. In Vivo. 1996. 10:483–488.16. Gao Y, Deng XG, Sun QN, Zhong ZQ. Ganoderma spore lipid inhibits N-methyl-N-nitrosourea-induced retinal photoreceptor apoptosis in vivo. Exp Eye Res. 2010. 90:397–404.17. Chen W, Yu M, Wang Y, Peng Y, Li X, Lam DM, Chen X, Liu X. Non-mitogenic human acidic fibroblast growth factor reduces retinal degeneration induced by sodium iodate. J Ocul Pharmacol Ther. 2009. 25:315–320.18. Kim IB, Kim KY, Joo CK, Lee MY, Oh SJ, Chung JW, Chun MH. Reaction of Müller cells after increased intraocular pressure in the rat retina. Exp Brain Res. 1998. 121:419–424.19. O'Callaghan JP. Assessment of neurotoxicity: use of glial fibrillary acidic protein as a biomarker. Biomed Environ Sci. 1991. 4:197–206.20. Seddon JM, Chen CA. The epidemiology of age-related macular degeneration. Int Ophthalmol Clin. 2004. 44:17–39.21. Bird AC. Therapeutic targets in age-related macular disease. J Clin Invest. 2010. 120:3033–3041.22. Pittler SJ, Baehr W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc Natl Acad Sci U S A. 1991. 88:8322–8326.23. Luhmann UF, Robbie S, Munro PM, Barker SE, Duran Y, Luong V, Fitzke FW, Bainbridge JW, Ali RR, MacLaren RE. The drusenlike phenotype in aging Ccl2-knockout mice is caused by an accelerated accumulation of swollen autofluorescent subretinal macrophages. Invest Ophthalmol Vis Sci. 2009. 50:5934–5943.24. Ohtaka K, Machida S, Ohzeki T, Tanaka M, Kurosaka D, Masuda T, Ishii T. Protective effect of hepatocyte growth factor against degeneration of the retinal pigment epithelium and photoreceptor in sodium iodate-injected rats. Curr Eye Res. 2006. 31:347–355.25. Mizota A, Adachi-Usami E. Functional recovery of retina after sodium iodate injection in mice. Vision Res. 1997. 37:1859–1865.26. Yamashita H, Yamasaki K, Sugihara K, Miyata H, Tsutsumi S, Iwaki Y. Full-field electroretinography obtained using a contact lens electrode with built-in high-intensity white-light-emitting diodes can be utilized in toxicological assessments in rats. Ophthalmic Res. 2009. 42:15–20.27. Smith SB, Hashimi W, Yielding KL. Retinal degeneration in the mouse induced transplacentally by N-methyl-N-nitrosourea: effects of constant illumination or total darkness. Exp Eye Res. 1988. 47:347–359.28. Kiuchi K, Kondo M, Ueno S, Moriguchi K, Yoshizawa K, Miyake Y, Matsumura M, Tsubura A. Functional rescue of N-methyl-N-nitrosourea-induced retinopathy by nicotinamide in Sprague-Dawley rats. Curr Eye Res. 2003. 26:355–362.29. Miki K, Uehara N, Shikata N, Matsumura M, Tsubura A. Poly (ADP-ribose) polymerase inhibitor 3-aminobenzamide rescues N-methyl-N-nitrosourea-induced photoreceptor cell apoptosis in Sprague-Dawley rats through preservation of nuclear factor-kappaB activity. Exp Eye Res. 2007. 84:285–292.30. Riggs LA. Electroretinography. Vision Res. 1986. 26:1443–1459.31. Tomita T, Yanagida T. Origins of the ERG waves. Vision Res. 1981. 21:1703–1707.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of a Post-vitrectomy Injection of N-methyl-N-nitrosourea as a Localized Retinal Degeneration Rabbit Model
- Nestin Expression in the Adult Mouse Retina with Pharmaceutically Induced Retinal Degeneration
- Early Reversible Changes on ERG in Pharmaceutically Induced Retinal Degeneration in Rats
- Activation of Caspase-3 During Photoreceptor Degeneration in rd Mouse Retina
- Expression Profiles of F4/80 and Nestin in Ocular Immune Cells Following Pharmaceutically Induced Retinal Degeneration in Adult Mice