Yonsei Med J.
2007 Jun;48(3):517-525. 10.3349/ymj.2007.48.3.517.
Activation of Intrarenal Complement System in Mouse Model for Chronic Cyclosporine Nephrotoxicity
- Affiliations
-
- 1Xenotransplantation Center, Division of Nephrology, Department of Internal Medicine, The Catholic University of Korea, Seoul, Korea. angch@catholic.ac.kr
- 2Department of Internal Medicine, The Affiliated Hospital, YanBian University Medical College, YanJi 133000, JiLin, PR China.
- 3Department of Laboratory Medicine, Hallym University College of Medicine, Anyang, Korea.
- 4Cell Death Research Center, Department of Anatomy, The Catholic University of Korea, Seoul, Korea.
- KMID: 724109
- DOI: http://doi.org/10.3349/ymj.2007.48.3.517
Abstract
- PURPOSE
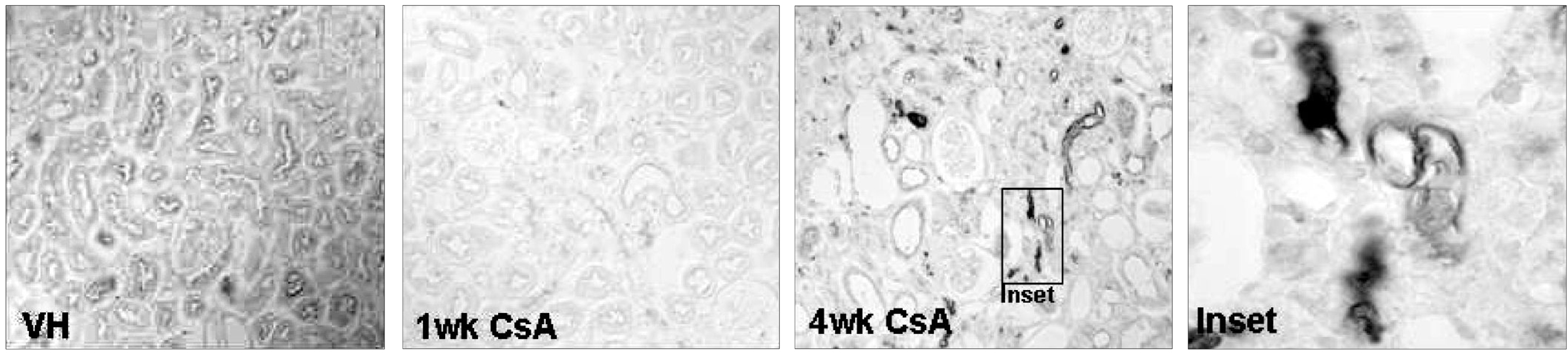
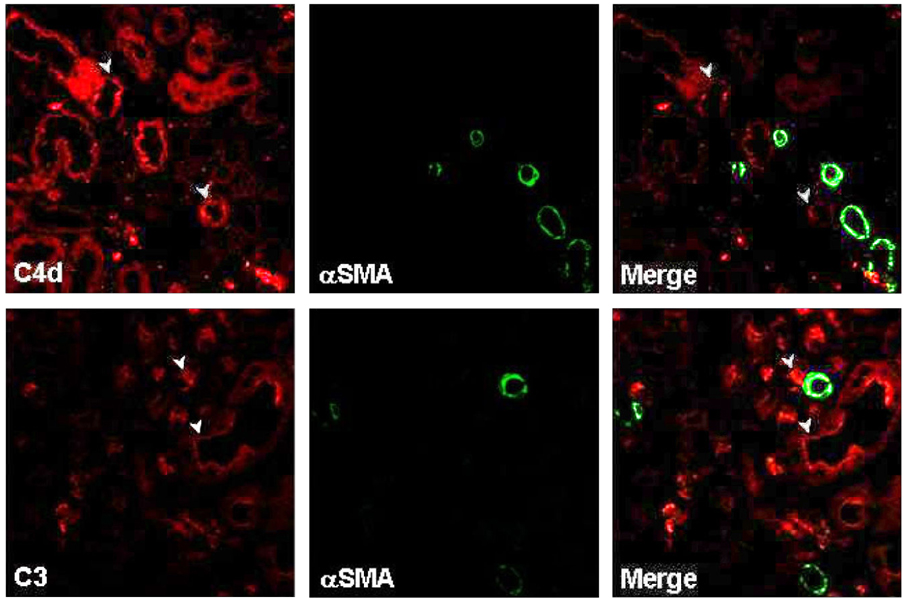
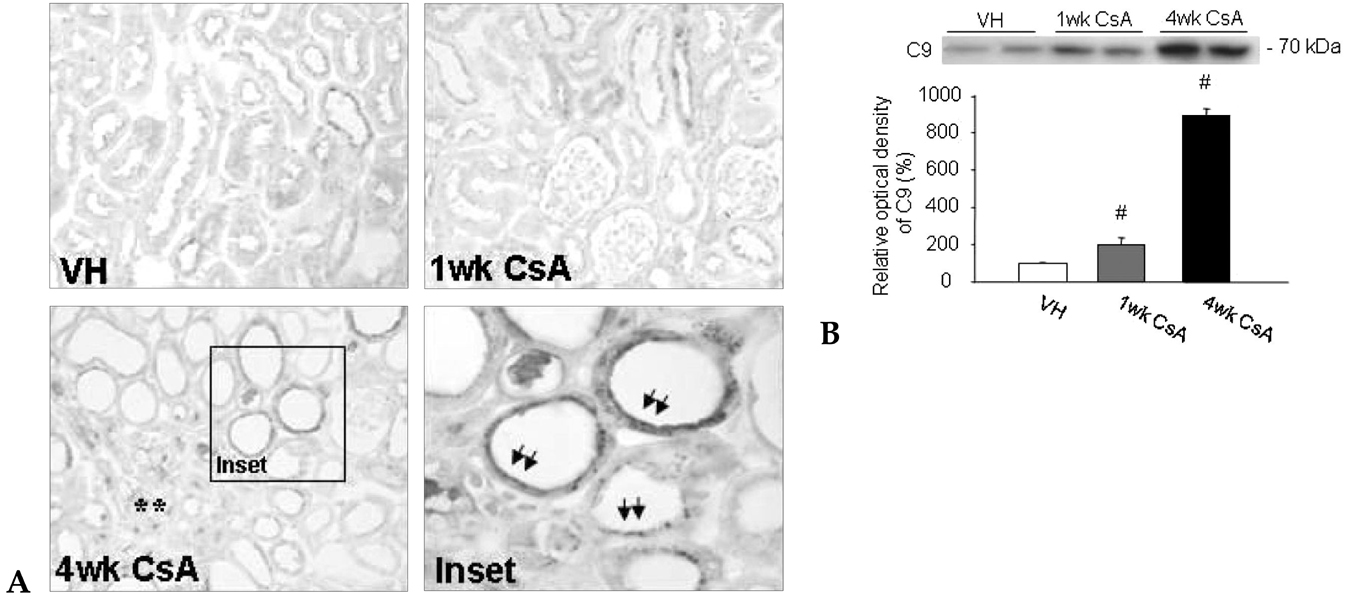
Local activation of the complement system plays a role in target organ damage. The aim of our study was to investigate the influence of cyclosporine (CsA)- induced renal injury on the complement system in the kidney. MATERIALS AND METHODS: Mice fed a low salt (0.01%) diet were treated with vehicle (VH, olive oil, 1mL/kg/day) or CsA (30mg/kg/day) for one or four weeks. Induction of chronic CsA nephrotoxicity was evaluated with renal function and histomorphology. Activation of the complement system was assessed through analysis of the expression of C3, C4d, and membrane attack complex (MAC), and the regulatory proteins, CD46 and CD55. CsA treatment induced renal dysfunction and typical morphology (tubulointerstitial inflammation and fibrosis) at four weeks. RESULTS: CsA-induced renal injury was associated with increased the expression of C3, C4d, and MAC (C9 and upregulation of complement regulatory proteins (CD 46 and CD55). Immunohistochemistry revealed that the activated complement components were mainly confined to the injured tubulointerstitium. CONCLUSION: CsA-induced renal injury is associated with activation of the intrarenal complement system.
Keyword
MeSH Terms
-
Animals
Antigens, CD45/analysis
Antigens, CD46/analysis
Antigens, CD55/analysis
Complement C3/analysis
Complement C4b/analysis
Complement Membrane Attack Complex/analysis
Complement System Proteins/*analysis
Cyclosporine/*toxicity
Disease Models, Animal
Immunity, Innate/drug effects
Immunoblotting
Immunohistochemistry
Immunosuppressive Agents/toxicity
Kidney/*drug effects/immunology/pathology
Kidney Diseases/*chemically induced/immunology
Mice
Microscopy, Confocal
Peptide Fragments/analysis
Figure
Reference
-
1. Walport MJ. Complement. First of two parts. N Engl J Med. 2001. 344:1058–1066.2. Walport MJ. Complement. Second of two parts. N Engl J Med. 2001. 344:1140–1144.3. Nangaku M, Shankland SJ, Couser WG. Cellular response to injury in membranous nephropathy. J Am Soc Nephrol. 2005. 16:1195–1204.
Article4. Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998. 97:2259–2267.
Article5. Yamada K, Miwa T, Liu J, Nangaku M, Song WC. Critical protection from renal ischemia reperfusion injury by CD55 and CD59. J Immunol. 2004. 172:3869–3875.
Article6. Sogabe H, Nangaku M, Ishibashi Y, Wada T, Fujita T, Sun X, et al. Increased susceptibility of decay-accelerating factor deficient mice to anti-glomerular basement membrane glomerulonephritis. J Immunol. 2001. 167:2791–2797.
Article7. Sacks SH, Zhou W, Andrews PA, Hartley B. Endogenous complement C3 synthesis in immune complex nephritis. Lancet. 1993. 342:1273–1274.
Article8. Hsu SI, Couser WG. Chronic progression of tubulointerstitial damage in proteinuric renal disease is mediated by complement activation: a therapeutic role for complement inhibitors? J Am Soc Nephrol. 2003. 14(7 Suppl 2):S186–S191.
Article9. Nangaku M, Pippin J, Couser WG. C6 mediates chronic progression of tubulointerstitial damage in rats with remnant kidneys. J Am Soc Nephrol. 2002. 13:928–936.
Article10. Nangaku M, Alpers CE, Pippin J, Shankland SJ, Kurokawa K, Adler S, et al. CD59 protects glomerular endothelial cells from immune-mediated thrombotic microangiopathy in rats. J Am Soc Nephrol. 1998. 9:590–597.
Article11. Qin X, Goldfine A, Krumrei N, Grubissich L, Acosta J, Chorev M, et al. Glycation inactivation of the complement regulatory protein CD59: a possible role in the pathogenesis of the vascular complications of human diabetes. Diabetes. 2004. 53:2653–2661.12. Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002. 8:582–587.
Article13. Li C, Lim SW, Sun BK, Yang CW. Chronic cyclosporine nephrotoxicity: new insights and preventive strategies. Yonsei Med J. 2004. 45:1004–1016.
Article14. Olyaei AJ, de Mattos AM, Bennett WM. Nephrotoxicity of immunosuppressive drugs: new insight and preventive strategies. Curr Opin Crit Care. 2001. 7:384–389.
Article15. Yang CW, Faulkner GR, Wahba IM, Christianson TA, Bagby GC, Jin DC, et al. Expression of apoptosis-related genes in chronic cyclosporine nephrotoxicity in mice. Am J Transplant. 2002. 2:391–399.
Article16. Mazzali M, Hughes J, Dantas M, Liaw L, Steitz S, Alpers CE, et al. Effects of cyclosporine in osteopontin null mice. Kidney Int. 2002. 62:78–85.17. Turnberg D, Botto M, Warren J, Morgan BP, Walport MJ, Cook HT. CD59a deficiency exacerbates accelerated nephrotoxic nephritis in mice. J Am Soc Nephrol. 2003. 14:2271–2279.
Article18. Tang S, Sheerin NS, Zhou W, Brown Z, Sacks SH. Apical proteins stimulate complement synthesis by cultured human proximal tubular epithelial cells. J Am Soc Nephrol. 1999. 10:69–76.
Article19. Welch TR, Beischel LS, Witte DP. Differential expression of complement C3 and C4 in the human kidney. J Clin Invest. 1993. 92:1451–1458.
Article20. Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Williams WW, Tolkoff-Rubin N, et al. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999. 10:2208–2214.21. Mroz A, Lewandowski Z, Cieciura T, Matlosz B, Pazik J, Kwiatkowski A, et al. C4d complement split product in diagnosis of immunological activity of chronic allograft nephropathy. Transplant Proc. 2006. 38:97–100.
Article22. Straatsburg IH, Boermeester MA, Wolbink GJ, van Gulik TM, Gouma DJ, Frederiks WM, et al. Complement activation induced by ischemia-reperfusion in humans: a study in patients undergoing partial hepatectomy. J Hepatol. 2000. 32:783–791.
Article23. Chang T, Chowdhry S, Budhu P, Kew RR. Smokeless tobacco extracts activate complement in vitro: a potential pathogenic mechanism for initiating inflammation of the oral mucosa. Clin Immunol Immunopathol. 1998. 87:223–229.
Article24. Nangaku M, Pippin J, Couser WG. Complement membrane attack complex (C5b-9) mediates interstitial disease in experimental nephrotic syndrome. J Am Soc Nephrol. 1999. 10:2323–2331.
Article25. Rangan GK, Pippin JW, Coombes JD, Couser WG. C5b-9 does not mediate chronic tubulointerstitial disease in the absence of proteinuria. Kidney Int. 2005. 67:492–503.
Article26. Endoh M, Yamashina M, Ohi H, Funahashi K, Ikuno T, Yasugi T, et al. Immunohistochemical demonstration of membrane cofactor protein (MCP) of complement in normal and diseased kidney tissues. Clin Exp Immunol. 1993. 94:182–188.
Article27. Cosio FG, Sedmak DD, Mahan JD, Nahman NS Jr. Localization of decay accelerating factor in normal and diseased kidneys. Kidney Int. 1989. 36:100–107.
Article28. Tamai H, Matsuo S, Fukatsu A, Nishikawa K, Sakamoto N, Yoshioka K, et al. Localization of 20-kD homologous restriction factor (HRF20) in diseased human glomeruli. An immunofluorescence study. Clin Exp Immunol. 1991. 84:256–262.
Article29. Lim SW, Li C, Ahn KO, Kim J, Moon IS, Ahn C, et al. Cyclosporine-induced renal injury induces toll-like receptor and maturation of dendritic cells. Transplantation. 2005. 80:691–699.
Article30. Li C, Yang CW, Park JH, Lim SW, Sun BK, Jung JY, et al. Pravastatin treatment attenuates interstitial inflammation and fibrosis in a rat model of chronic cyclosporine-induced nephropathy. Am J Physiol Renal Physiol. 2004. 286:F46–F57.
Article31. Shagdarsuren E, Wellner M, Braesen JH, Park JK, Fiebeler A, Henke N, et al. Complement activation in angiotensin II-induced organ damage. Circ Res. 2005. 97:716–724.
Article32. Pasch MC, Van Den Bosch NH, Daha MR, Bos JD, Asghar SS. Synthesis of complement components C3 and factor B in human keratinocytes is differentially regulated by cytokines. J Invest Dermatol. 2000. 114:78–82.
Article33. Lee EK, Kang SM, Paik DJ, Kim JM, Youn J. Essential roles of Toll-like receptor-4 signaling in arthritis induced by type II collagen antibody and LPS. Int Immunol. 2005. 17:325–333.
Article34. Xin J, Homma T, Matsusaka T, Ma J, Isaka Y, Imai E, et al. Suppression of cyclosporine a nephrotoxicity in vivo by transforming growth factor beta receptor-immunoglobulin G chimeric protein. Transplantation. 2004. 77:1433–1442.
Article35. Lee CT, Huynh VM, Lai LW, Lien YH. Cyclosporine A-induced hypercalciuria in calbindin-D28k knockout and wild-type mice. Kidney Int. 2002. 62:2055–2061.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chronic Cyclosporine Nephrotoxicity: New Insights and Preventive Strategies
- A Case of Chronic Idiopathic Urticaria Treated with Cyclosporine
- Chronic Cyclosporine Nephrotoxicity in Idiopathic Nephrotic Syndrome of Childhood
- Effectiveness of Cyclosporine in a 10-year-old Girl with C3 Glomerulopathy
- Effects of Cyclosporine on the Intrarenal Renin-Angiotensin System