Endocrinol Metab.
2021 Aug;36(4):865-874. 10.3803/EnM.2021.1108.
Aldosterone Inhibits In Vitro Myogenesis by Increasing Intracellular Oxidative Stress via Mineralocorticoid Receptor
- Affiliations
-
- 1Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Division of Endocrinology and Metabolism, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2519674
- DOI: http://doi.org/10.3803/EnM.2021.1108
Abstract
- Background
Despite clinical evidence indicating poor muscle health in subjects with primary aldosteronism (PA), it is still unclear whether the role of aldosterone in muscle metabolism is direct or mediated indirectly via factors, such as electrolyte imbalance or impaired glucose uptake. As one approach to clarify this issue, we investigated the effect of aldosterone on in vitro myogenesis and the potential mechanism explaining it.
Methods
Myogenesis was induced in mouse C2C12 myoblasts with 2% horse serum. Immunofluorescence, quantitative reversetranscription polymerase chain reaction, Western blot, viability, and migration analyses were performed for experimental research.
Results
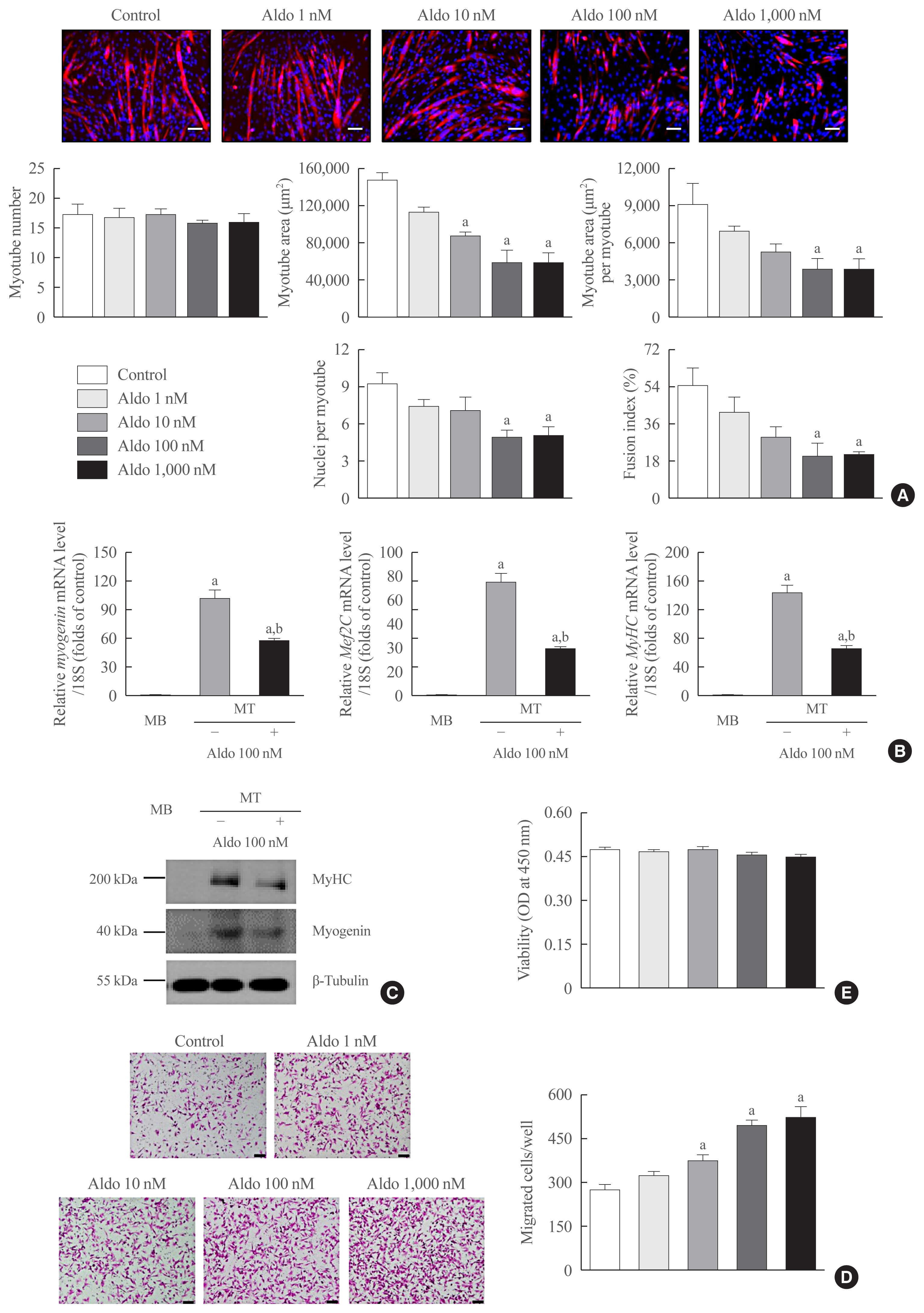
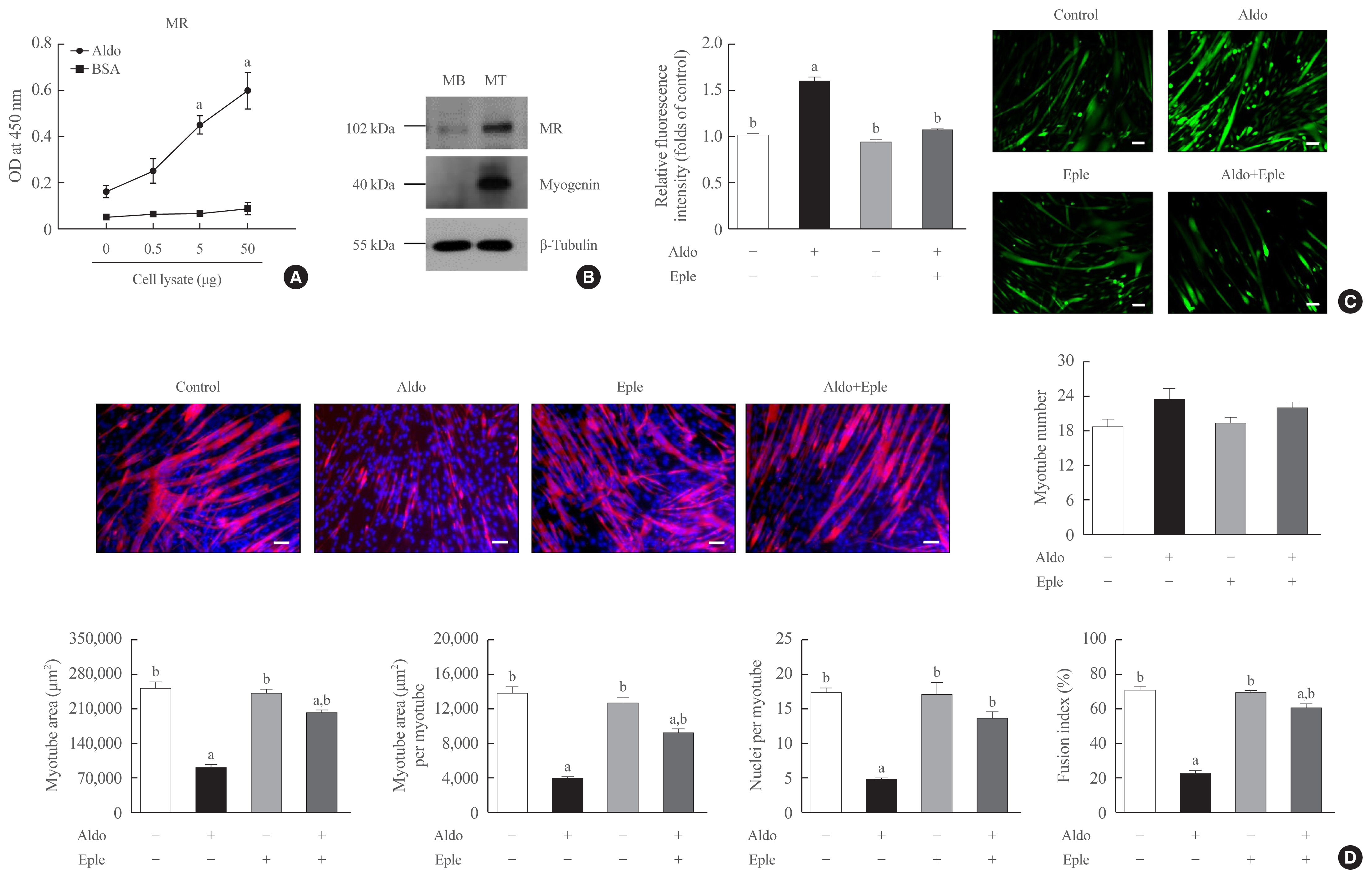
Recombinant aldosterone treatment suppressed muscle differentiation from mouse C2C12 myoblasts in a dose-dependent manner, and consistently reduced the expression of myogenic differentiation markers. Furthermore, aldosterone significantly increased intracellular reactive oxygen species (ROS) levels in myotubes, and treatment with N-acetyl cysteine, a potent biological thiol antioxidant, reversed the decrease of myotube area, myotube area per myotube, nucleus number per myotube, and fusion index due to aldosterone through decreasing oxidative stress. A binding enzyme-linked immunosorbent assay confirmed that mineralocorticoid receptor (MR) interacted with aldosterone in C2C12 myoblasts, while eplerenone, an MR inhibitor, blocked aldosterone-stimulated intracellular ROS generation during myogenesis and markedly attenuated the suppression of in vitro myogenesis by aldosterone.
Conclusion
These findings support the hypothesis that hypersecretion of aldosterone, like PA, directly contributes to muscular deterioration and suggest that antioxidants and/or MR antagonists could be effective therapeutic options to reduce the risk of sarcopenia in these patients.
Figure
Reference
-
1. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019; 393:2636–46.
Article2. Dao T, Green AE, Kim YA, Bae SJ, Ha KT, Gariani K, et al. Sarcopenia and muscle aging: a brief overview. Endocrinol Metab (Seoul). 2020; 35:716–32.
Article3. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48:16–31.
Article4. Kim YK, Yi SR, Lee YH, Kwon J, Jang SI, Park SH. Effect of sarcopenia on postoperative mortality in osteoporotic hip fracture patients. J Bone Metab. 2018; 25:227–33.
Article5. Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016; 7:512–4.
Article6. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997; 127(5 Suppl):990S–991S.
Article7. Kim SH, Shin MJ, Shin YB, Kim KU. Sarcopenia associated with chronic obstructive pulmonary disease. J Bone Metab. 2019; 26:65–74.
Article8. Tevosian SG, Fox SC, Ghayee HK. Molecular mechanisms of primary aldosteronism. Endocrinol Metab (Seoul). 2019; 34:355–66.
Article9. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016; 101:1889–916.
Article10. Chen ZW, Hung CS, Wu VC, Lin YH. TAIPAI study group. Primary aldosteronism and cerebrovascular diseases. Endocrinol Metab (Seoul). 2018; 33:429–34.
Article11. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005; 45:1243–8.
Article12. Burniston JG, Saini A, Tan LB, Goldspink DF. Aldosterone induces myocyte apoptosis in the heart and skeletal muscles of rats in vivo. J Mol Cell Cardiol. 2005; 39:395–9.
Article13. Kwak MK, Lee SE, Cho YY, Suh S, Kim BJ, Song KH, et al. The differential effect of excess aldosterone on skeletal muscle mass by sex. Front Endocrinol (Lausanne). 2019; 10:195.
Article14. Kwak MK, Lee JY, Kim BJ, Lee SH, Koh JM. Effects of primary aldosteronism and different therapeutic modalities on glucose metabolism. J Clin Med. 2019; 8:2194.
Article15. Aagaard NK, Andersen H, Vilstrup H, Clausen T, Jakobsen J, Dorup I. Muscle strength, Na, K-pumps, magnesium and potassium in patients with alcoholic liver cirrhosis: relation to spironolactone. J Intern Med. 2002; 252:56–63.
Article16. Lastra G, Whaley-Connell A, Manrique C, Habibi J, Gutweiler AA, Appesh L, et al. Low-dose spironolactone reduces reactive oxygen species generation and improves insulin-stimulated glucose transport in skeletal muscle in the TG(mRen2)27 rat. Am J Physiol Endocrinol Metab. 2008; 295:E110–6.
Article17. Kim DA, Park SJ, Lee JY, Kim JH, Lee S, Lee E, et al. Effect of CCL11 on in vitro myogenesis and its clinical relevance for sarcopenia in older adults. Endocrinol Metab (Seoul). 2021; 36:455–65.
Article18. Park SJ, Lee JY, Lee SH, Koh JM, Kim BJ. SLIT2 inhibits osteoclastogenesis and bone resorption by suppression of Cdc42 activity. Biochem Biophys Res Commun. 2019; 514:868–74.
Article19. Lee JY, Park SJ, Kim DA, Lee SH, Koh JM, Kim BJ. Muscle-derived lumican stimulates bone formation via integrin α2β1 and the downstream ERK signal. Front Cell Dev Biol. 2020; 8:565826.
Article20. Kim BJ, Lee YS, Lee SY, Baek WY, Choi YJ, Moon SA, et al. Osteoclast-secreted SLIT3 coordinates bone resorption and formation. J Clin Invest. 2018; 128:1429–41.
Article21. Gorini S, Kim SK, Infante M, Mammi C, La Vignera S, Fabbri A, et al. Role of aldosterone and mineralocorticoid receptor in cardiovascular aging. Front Endocrinol (Lausanne). 2019; 10:584.
Article22. Inthachart K, Manotham K, Eiam-Ong S, Eiam-Ong S. Aldosterone rapidly enhances levels of the striatin and caveolin-1 proteins in rat kidney: the role of the mineralocorticoid receptor. Endocrinol Metab (Seoul). 2019; 34:291–301.
Article23. Szentesi P, Csernoch L, Dux L, Keller-Pinter A. Changes in redox signaling in the skeletal muscle with aging. Oxid Med Cell Longev. 2019; 2019:4617801.
Article24. Powers SK, Morton AB, Ahn B, Smuder AJ. Redox control of skeletal muscle atrophy. Free Radic Biol Med. 2016; 98:208–17.
Article25. Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002; 82:47–95.
Article26. Abrigo J, Elorza AA, Riedel CA, Vilos C, Simon F, Cabrera D, et al. Role of oxidative stress as key regulator of muscle wasting during cachexia. Oxid Med Cell Longev. 2018; 2018:2063179.27. Mecocci P, Fano G, Fulle S, MacGarvey U, Shinobu L, Polidori MC, et al. Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic Biol Med. 1999; 26:303–8.
Article28. Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, et al. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol. 2007; 293:H2009–23.
Article29. Fanelli C, Zatz R. Linking oxidative stress, the renin-angiotensin system, and hypertension. Hypertension. 2011; 57:373–4.
Article30. Brown NJ. Aldosterone and vascular inflammation. Hypertension. 2008; 51:161–7.
Article31. Hawkins UA, Gomez-Sanchez EP, Gomez-Sanchez CM, Gomez-Sanchez CE. The ubiquitous mineralocorticoid receptor: clinical implications. Curr Hypertens Rep. 2012; 14:573–80.
Article32. Gomez-Sanchez EP. Brain mineralocorticoid receptors: orchestrators of hypertension and end-organ disease. Curr Opin Nephrol Hypertens. 2004; 13:191–6.
Article33. Chadwick JA, Hauck JS, Lowe J, Shaw JJ, Guttridge DC, Gomez-Sanchez CE, et al. Mineralocorticoid receptors are present in skeletal muscle and represent a potential therapeutic target. FASEB J. 2015; 29:4544–54.
Article34. Delyani JA, Rocha R, Cook CS, Tobert DS, Levin S, Roniker B, et al. Eplerenone: a selective aldosterone receptor antagonist (SARA). Cardiovasc Drug Rev. 2001; 19:185–200.
Article35. Kim BJ, Lee SH, Koh JM. Bone health in adrenal disorders. Endocrinol Metab (Seoul). 2018; 33:1–8.
Article36. Kim BJ, Kwak MK, Ahn SH, Kim H, Lee SH, Koh JM. Lower trabecular bone score in patients with primary aldosteronism: human skeletal deterioration by aldosterone excess. J Clin Endocrinol Metab. 2018; 103:615–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pseudohypoaldosteronism Type 1
- The Role of Oxidative Stress in the Pathogenesis of Asthma
- Hypertensive Hypokalemic Disorders
- Aldosterone Rapidly Enhances Levels of the Striatin and Caveolin-1 Proteins in Rat Kidney: The Role of the Mineralocorticoid Receptor
- Aldosterone Upregulates Connective Tissue Growth Factor Gene Expression via p38 MAPK Pathway and Mineralocorticoid Receptor in Ventricular Myocytes