J Korean Med Sci.
2004 Dec;19(6):805-811. 10.3346/jkms.2004.19.6.805.
Aldosterone Upregulates Connective Tissue Growth Factor Gene Expression via p38 MAPK Pathway and Mineralocorticoid Receptor in Ventricular Myocytes
- Affiliations
-
- 1Department of Medicine, Samsung Medical Center, Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Seoul, Korea. dkkim@smc.samsung.co.kr
- KMID: 1778560
- DOI: http://doi.org/10.3346/jkms.2004.19.6.805
Abstract
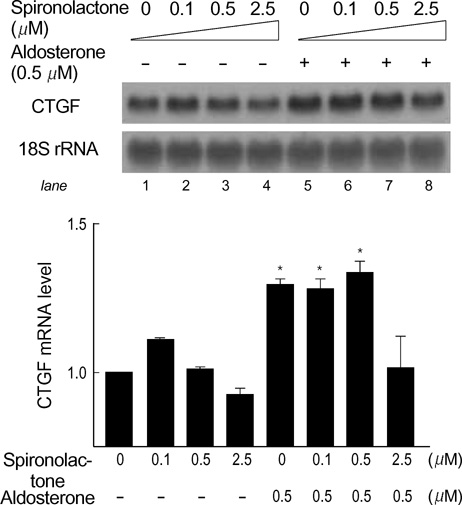
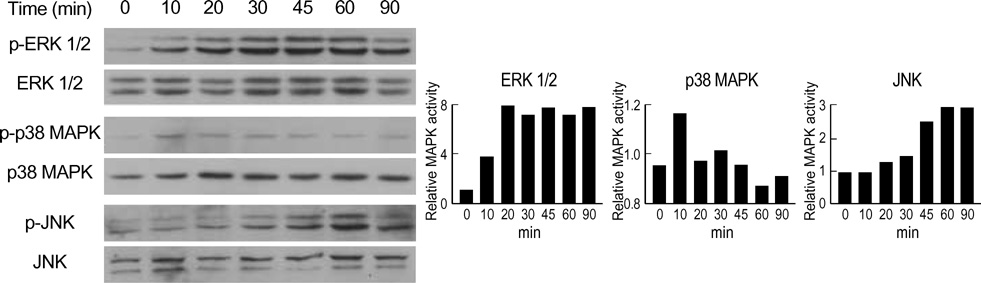
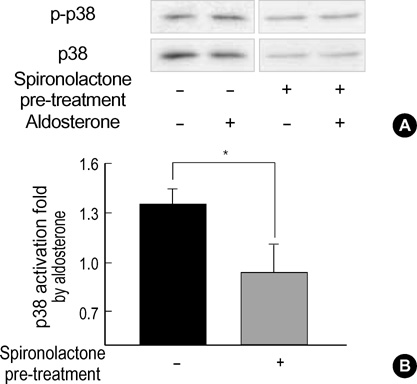
- The effect of aldosterone on connective tissue growth factor (CTGF) was examined in rat embryonic ventricular myocytes. Upon aldosterone treatment, CTGF expression was significantly increased in a dose and time-dependent manner. To explore the molecular mechanism for this upregulation, we examined the role of mineralocorticoid receptor. Pre-treatment of an antagonist (spironolactone) at 5-fold excess of aldosterone blocked the CTGF induction by aldosterone, suggesting that the upregulation was mediated by mineralocorticoid receptor. Aldosterone treatment resulted in activation of ERK1/2, p38 MAPK, and JNK pathways with a more transient pat-tern in p38 MAPK. Blocking studies using pre-treatment of the inhibitor of each path-way revealed that p38 MAPK cascade may be important for aldosterone-mediated CTGF upregulation as evidenced by the blocking of CTGF induction by SB203580 (p38 MAPK inhibitor), but not by PD098059 (ERK1/2 inhibitor) and JNK inhibitor I. Interestingly, JNK inhibitor I and PD098059 decreased the basal level of CTGF expression. On the other hand, pre-treatment of spironolactone abrogated the p38 MAPK activation, indicating that mineralocorticoid receptor mechanism is linked to p38 MAPK pathway. Taken together, our findings suggest that aldosterone induces CTGF expression via both p38 MAPK cascade and mineralocorticoid receptor and that cross-talk exists between the two pathways.
Keyword
MeSH Terms
-
Aldosterone/*pharmacology
Animals
Cells, Cultured
Dose-Response Relationship, Drug
Gene Expression Regulation/drug effects/physiology
Heart Ventricles/drug effects/embryology/metabolism
Immediate-Early Proteins/*metabolism
Intercellular Signaling Peptides and Proteins/*metabolism
Myocytes, Cardiac/*drug effects/*metabolism
Rats
Receptors, Mineralocorticoid/*metabolism
Research Support, Non-U.S. Gov't
Signal Transduction/drug effects/physiology
Spironolactone/pharmacology
Up-Regulation/drug effects/physiology
p38 Mitogen-Activated Protein Kinases/*metabolism
Figure
Cited by 1 articles
-
Expression of NAD(P)H Oxidase Subunits and Their Contribution to Cardiovascular Damage in Aldosterone/Salt-Induced Hypertensive Rat
Young Mee Park, Bong Hee Lim, Rhian M. Touyz, Jeong Bae Park
J Korean Med Sci. 2008;23(6):1039-1045. doi: 10.3346/jkms.2008.23.6.1039.
Reference
-
1. Young M, Funder JW. Aldosterone and the heart. Trends Endocrinol Metab. 2000. 11:224–226.
Article2. Robert V, van Thiem N, Cheav SL, Mouas C, Swynghedauw B, Delcayre C. Increased cardiac types I and III collagen mRNAs in aldosterone-salt hypertension. Hypertension. 1994. 24:30–36.
Article3. Robert V, Silvestre JS, Charlemagne D, Sabri A, Trouve P, Wassef M, Swynghedauw B, Delcayre C. Biological determinants of aldosterone-induced cardiac fibrosis in rats. Hypertension. 1995. 26:971–978.
Article4. Brilla CG, Zhou G, Matsubara L, Weber KT. Collagen metabolism in cultured adult rat cardiac fibroblasts: response to angiotensin II and aldosterone. J Mol Cell Cardiol. 1994. 26:809–820.
Article5. Fullerton MJ, Funder JW. Aldosterone and cardiac fibrosis: in vitro studies. Cardiovasc Res. 1994. 28:1863–1867.
Article6. Zhou G, Kandala JC, Tyagi SC, Katwa LC, Weber KT. Effects of angiotensin II and aldosterone on collagen gene expression and protein turnover in cardiac fibroblasts. Mol Cell Biochem. 1996. 154:171–178.
Article7. Stockand JD, Meszaros JG. Aldosterone stimulates proliferation of cardiac fibroblasts by activating Ki-RasA and MAPK1/2 signaling. Am J Physiol-Heart Circ Physiol. 2003. 284:H176–H184.8. Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001. 345:1689–1697.
Article9. Silvestre JS, Robert V, Heymes C, Aupetit-Faisant B, Mouas C, Moalic JM, Swynghedauw B, Delcayre C. Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J Biol Chem. 1998. 273:4883–4891.10. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999. 341:709–717.11. Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: A cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991. 114:1285–1294.
Article12. Kothapalli D, Frazier KS, Welply A, Segarini PR, Grotendorst GR. Transforming growth factor beta induces anchorage-independent growth of NRK fibroblasts via a connective tissue growth factor-dependent signaling pathway. Cell Growth Differ. 1997. 8:61–68.13. Nakanishi T, Kimura Y, Tamura T, Ichikawa H, Yamaai Y, Sugimoto T, Takigawa M. Cloning of a mRNA preferentially expressed in chondrocytes by differential display-PCR from a human chondrocytic cell line that is identical with connective tissue growth factor (CTGF) mRNA. Biochem Biophys Res Commun. 1997. 234:206–210.
Article14. Hishikawa K, Nakaki T, Fujii T. Transforming growth factor-beta (1) induces apoptosis via connective tissue growth factor in human aortic smooth muscle cells. Eur J Pharmacol. 1999. 385:287–290.15. Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999. 20:189–206.16. Lasky JA, Ortiz LA, Tonthat B, Hoyle GW, Corti M, Athas G, Lungarella G, Brody A, Friedman M. Connective tissue growth factor mRNA expression is upregulated in bleomycin-induced lung fibrosis. Am J Physiol - Lung Cell Mol Physiol. 1998. 275:L365–L371.17. Ito Y, Aten J, Bende RJ, Oemar BS, Rabelink TJ, Weening JJ, Goldschmeding R. Expression of connective tissue growth factor in human renal fibrosis. Kidney Int. 1998. 53:853–861.
Article18. Paradis V, Dargere D, Vidaud M, De Gouville AC, Huet S, Martinez V, Gauthier JM, Ba N, Sobesky R, Ratziu V, Bedossa P. Expression of connective tissue growth factor in experimental rat and human liver fibrosis. Hepatology. 1999. 30:968–976.
Article19. Igarashi A, Nashiro K, Kikuchi K, Sato S, Ihn H, Fujimoto M, Grotendorst GR, Takehara K. Connective tissue growth factor gene expression in tissue sections from localized scleroderma, keloid, and other fibrotic skin disorders. J Invest Dermatol. 1996. 106:729–733.
Article20. Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997. 8:171–179.21. Ohnishi H, Oka T, Kusachi S, Nakanishi T, Takeda K, Nakahama M, Doi M, Murakami T, Ninomiya Y, Takigawa M, Tsuji T. Increased expression of connective tissue growth factor in the infarct zone of experimentally induced myocardial infarction in rats. J Mol Cell Cardiol. 1998. 30:2411–2422.
Article22. Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF-beta in cardiac fibroblasts and cardiac myocytes: A potential role in heart fibrosis. J Mol Cell Cardiol. 2000. 32:1805–1819.23. Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003. 63:1791–1800.
Article24. Brilla CG, Matsubara LS, Weber KT. Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J Mol Cell Cardiol. 1993. 25:563–575.
Article25. Nicoletti A, Heudes D, Hinglais N, Appay MD, Philippe M, Sassy-Prigent C, Bariety J, Michel JB. Left ventricular fibrosis in renovascular hypertensive rats. Effect of losartan and spironolactone. Hypertension. 1995. 26:101–111.26. Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993. 268:14553–14556.
Article27. Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995. 270:27489–27494.
Article28. Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995. 364:229–233.29. Barr RK, Kendrick TS, Bogoyevitch MA. Identification of the critical features of a small peptide inhibitor of JNK activity. J Biol Chem. 2002. 277:10987–10997.
Article30. Gumz ML, Popp MP, Wingo CS, Cain BD. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol - Renal Physiol. 2003. 285:F664–F673.31. Cohen P. Dissection of protein kinase cascades that mediate cellular response to cytokines and cellular stress. Adv Pharmacol. 1996. 36:15–27.
Article32. English J, Pearson G, Wilsbacher J, Swantek J, Karandikar M, Xu S, Cobb MH. New insights into the control of MAP kinase pathways. Exp Cell Res. 1999. 253:255–270.
Article33. Matsuoka H, Arai T, Mori M, Goya S, Kida H, Morishita H, Fujiwara H, Tachibana I, Osaki T, Hayahi S. A p38 MAPK inhibitor, FR-167653, ameliorates murine bleomycin-induced pulmonary fibrosis. Am J Physiol - Lung Cell Mol Physiol. 2002. 283:L103–L112.
Article34. Oshima Y, Fujio Y, Funamoto M, Negoro S, Izumi M, Nakaoka Y, Hirota H, Yamauchi-Takihara K, Kawase I. Aldosterone augments endothelin-1-induced cardiac myocyte hypertrophy with the reinforcement of the JNK pathway. FEBS Lett. 2002. 524:123–126.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of aldosterone on epithelial-to-mesenchymal transition of human peritoneal mesothelial cells
- Aldosterone Modulates Cell Proliferation and Apoptosis in the Neonatal Rat Heart
- Delay of Spontaneous Neutrophil Apoptosis by Vascular Endothelial Growth Factor
- Hypoxia Induces Connective Tissue Growth Factor mRNA Expression
- Effect of Eplerenone, a Selective Aldosterone Blocker, on the Development of Diabetic Nephropathy in Type 2 Diabetic Rats