Korean Lepr Bull.
2019 Dec;52(1):29-40. 10.33161/klb.2019.52.1.29.
Comparison of Effective DNA Extraction method for Molecular biological study of Mycobacterium leprae
- Affiliations
-
- 1Institute for Leprosy Research, Korean Hansen Welfare Association, Korea. yunjipod@naver.com
- KMID: 2466490
- DOI: http://doi.org/10.33161/klb.2019.52.1.29
Abstract
- BACKGROUND
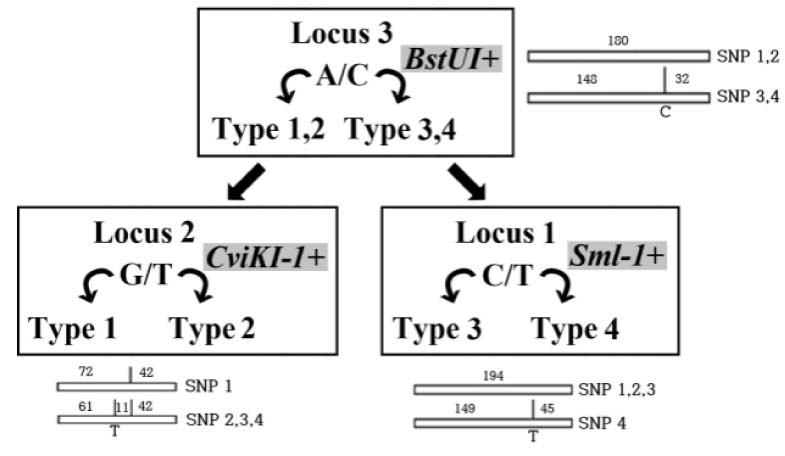
Leprosy is an important health problem in many geographical areas yet. It is caused through a cough or contact with fluid from the nose of a person infected by Mycobacterium leprae. Study of DNA from M. leprae is important to understand essentiality for leprosy. However, there is no standard in many parts, so various studies are needed. OBJECTS: In this study, DNA extraction method were confirmed for the effective detection of M. leprae. And restriction enzyme fragment length polymorphism typing and high resolution melt (HRM) analysis were performed for comparison with sequencing analysis.
METHODS
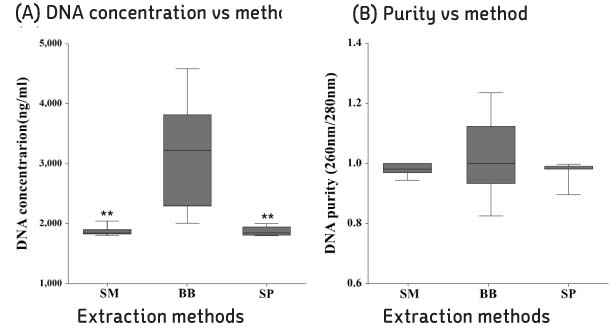
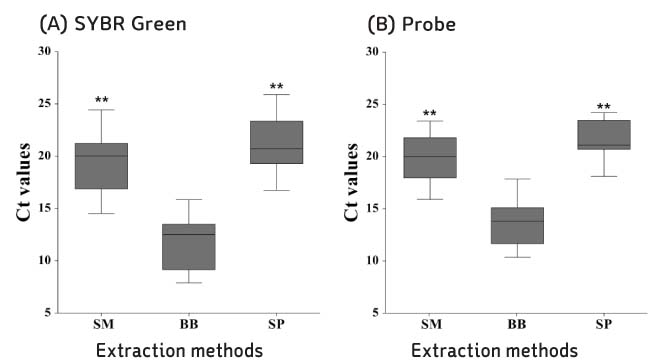
Compared with three DNA extraction methods (BB, SM and SP) with real-time polymerase chain reaction (PCR). Analysis single nucleotide polymorphism (SNP) genotype and tandem repeats by PCR amplification, and then compare with sequence.
RESULTS
BB method was effective when measuring the concentration and threshold cycle (Ct) compared with SM and SP methods. When compared with restriction fragment length polymorphism typing method and sequence analysis, all methods were suitable for SNP1 and 3 type classification. Tandem repeats values of BB method were correspond to sequence analysis than SM and SP methods in HRM analysis.
CONCLUSIONS
The DNA extraction method by bead is useful approach for studying of M. leprae.
Keyword
MeSH Terms
Figure
Reference
-
1. Cardona Castro N, BeltránAlzate JC, Romero-Montoya IM, Meléndez E, Torres F, Sakamuri RM. Identification and comparison of Mycobacterium leprae genotypes in two geographical regions of Colombia. Lepr Rev. 2009; 80(3):316–320.2. Suzuki K, Akama T, Kawashima A, Yoshihara A, Yotsu RR, Ishii N. Current status of leprosy: epidemiology, basic science and clinical perspectives. J Dermatol. 2012; 39(2):121–129.
Article3. Suzuki K, Takigawa W, Tanigawa K, Nakamura K, Ishido Y, Kawashima A. Detection of Mycobacterium leprae DNA from Archaeological Skeletal Remains in Japan Using Whole Genome Amplification and Polymerase Chain Reaction. PLoS One. 2010; 26(5):e12422.4. Martinez AN, Lahiri R, Pittman TL, Scollard D, Truman R, Moraes MO. Molecular determination of Mycobacterium leprae viability by use of Real-time PCR. J Clin Microbiol. 2009; 47(7):2124–2130.
Article5. Plikaytis BB, Gelber RH, Shinnick TM. Rapid and sensitive detection of Mycobacterium leprae using a nested-primer gene amplification assay. J Clin Microbiol. 1990; 28:1913–1191.
Article6. World Health Organization(WHO). Guidelines for the Diagnosis, Treatment and Prevention of Lepros. 2018. ISBN: 978 92 9022 638 3.7. Nakamura M. Elimination of contaminants in a homogenate of nude-mouse footpad experimentally infected with Mycobacterium leprae. Nihon Rai Gakkai Zasshi. 1994; 63(2):47–50.
Article8. Monot M, Honoré N, Garnier T, Araoz R, Coppée JY, Lacroix C. On the Origin of Leprosy. Science. 2005; 308:1040–1104.
Article9. Sakamuri RM, Kimura M, Li W, Kim HC, Lee H, Kiran MD. Population-based molecular epidemiology of leprosy in Cebu. Philippines. J Clin Microbiol. 2009; 47(9):2844–2854.
Article10. Matsuoka M, Maeda S, Kai M, Nakata N, Chae GT, Gillis TP. Mycobacterium leprae typing by genomic diversity and global distribution of genotypes. Int J Lepr Other Mycobact Dis. 2000; 68(2):121–128.11. Shepard C. The experimental disease that follows the injection of human leprosy bacillus into footpads of mice. J Exp Med. 1960; 112:445–445.12. Williams DL, Gillis TP, Booth RJ, Looker D, Watson JD. The use of a specific DNA probe and polymerase chain reaction for the detection of Mycobacterium leprae. J Infect Dis. 1990; 162(1):193–200.
Article13. Hart skeerl RA, De Wit MYL, Klatser PR. Polymerase chain reaction for the detection of Mycobacterium leprae. J Gen Microbiol. 1989; 135(9):2357–2364.14. Masuoka M, Lopez R, Budiawan T, Kyaw K, Chae GT. Genotypic analysis of Mycobacterium leprae isolates from Japan and other Asian countries reveals a global transmission pattern of leprosy. FEMS Microbiol Let. 2006; 261(1):150–154.15. Weng X, Wang Z, Liu J, Kimura M, Black WC 4th, Brennan PJ. Identification and distribution of Mycobacterium leprae genotypes in a region of high leprosy prevalence in China. J Clin Microbiol. 2007; 45(6):1728–1734.
Article16. Matsuoka M, Zhang L. Molecular epidemiology of the leprosy. Nihon Hansenbyo Gakkai Zasshi. 2004; 73(1):7–14.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rapid detection of Mycobacterium leprae for the diagnosis of leprosy: comparison between loop-mediated isothermal amplification and PCR.
- The usefulness of multiple displacement amplification in the molecular studies in Hansen's disease
- Molecular Epidemiology of Mycobacterium leprae as Determined by Structure-Neighbor Clustering in Korea found cases
- Detection of Mycobacterium leprae in Skin Biopsy Sepcimens From Leprosy patients by Polymerase Chain Reaction
- Possibility of Zebrafish as New infection model for Leprosy