Clin Exp Otorhinolaryngol.
2019 Nov;12(4):367-375. 10.21053/ceo.2018.01298.
Response of Glucocorticoid Receptor Alpha and Histone Deacetylase 2 to Glucocorticoid Treatment Predicts the Prognosis of Sudden Sensorineural Hearing Loss
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Nanjing Drum Tower Hospital Clinical College of Nanjing Medical University, Nanjing City, China. shewandong@163.com
- 2Department of Otolaryngology-Head and Neck Surgery, The Affiliated Jiangyin Hospital of Southeast University Medical School, Jiangyin City, China.
- 3Department of Otolaryngology-Head and Neck Surgery, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing City, China.
- KMID: 2462732
- DOI: http://doi.org/10.21053/ceo.2018.01298
Abstract
OBJECTIVES
To investigate glucocorticoid receptor (GR) and histone deacetylase 2 (HDAC2) gene expression and protein levels in peripheral blood mononuclear cells (PBMCs) of patients with severe or profound sudden sensorineural hearing loss (SSNHL) and to explore the roles of GRs and HDAC2 in glucocorticoid (GC) insensitivity.
METHODS
Fifty-five severe or profound SSNHL patients were enrolled in the study. According to hearing improvement after GC treatment, patients were assigned into two groups: GC-sensitive and GC-resistant. A normal reference group included 20 healthy volunteers without hearing loss. Quantitative real-time polymerase chain reaction and Western blot analyses were used to detect the relative expression of GRα, GRβ, and HDAC2 in PBMCs at the mRNA and protein levels.
RESULTS
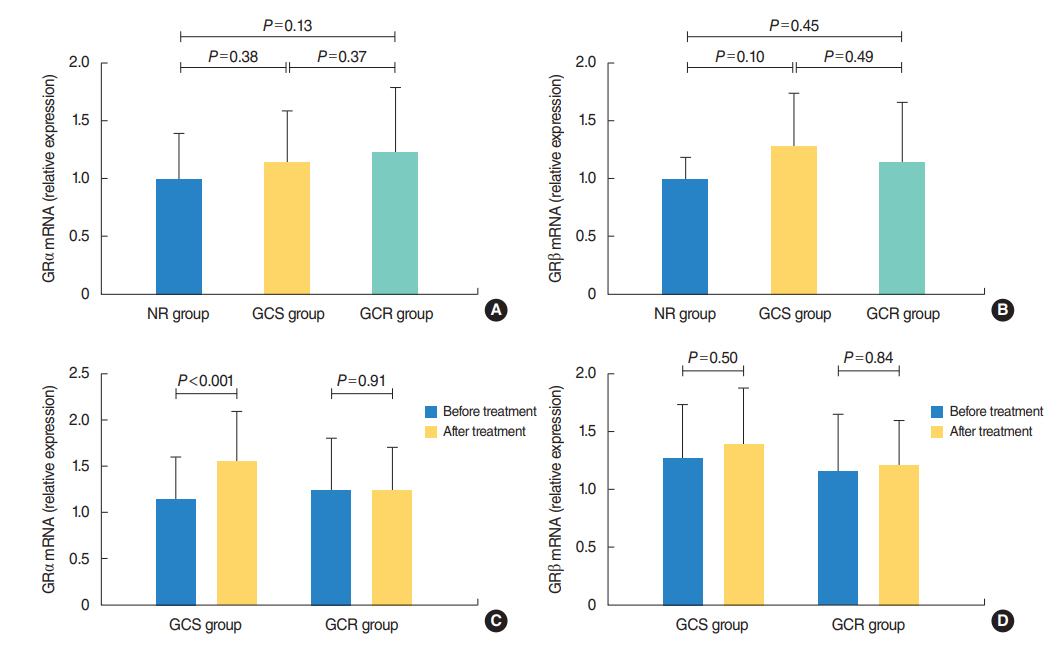
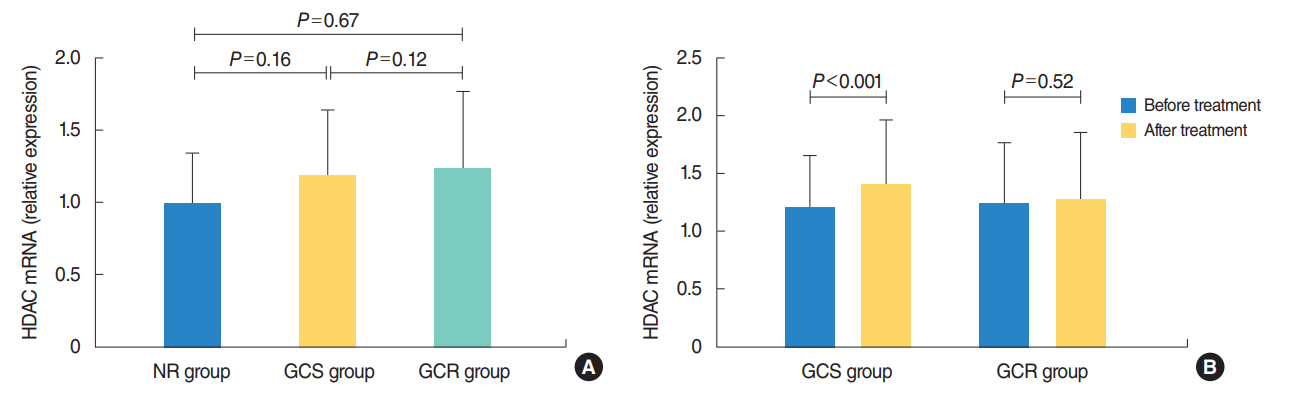
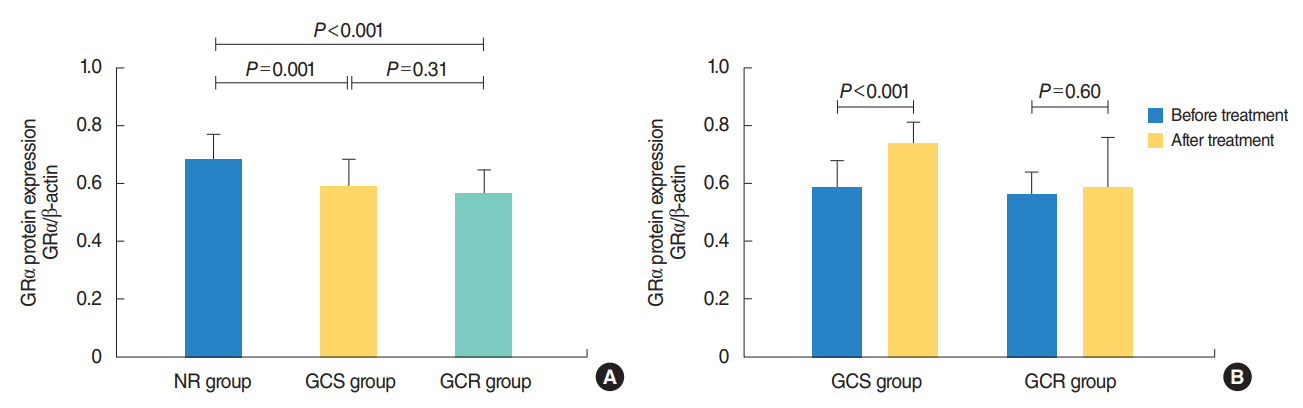
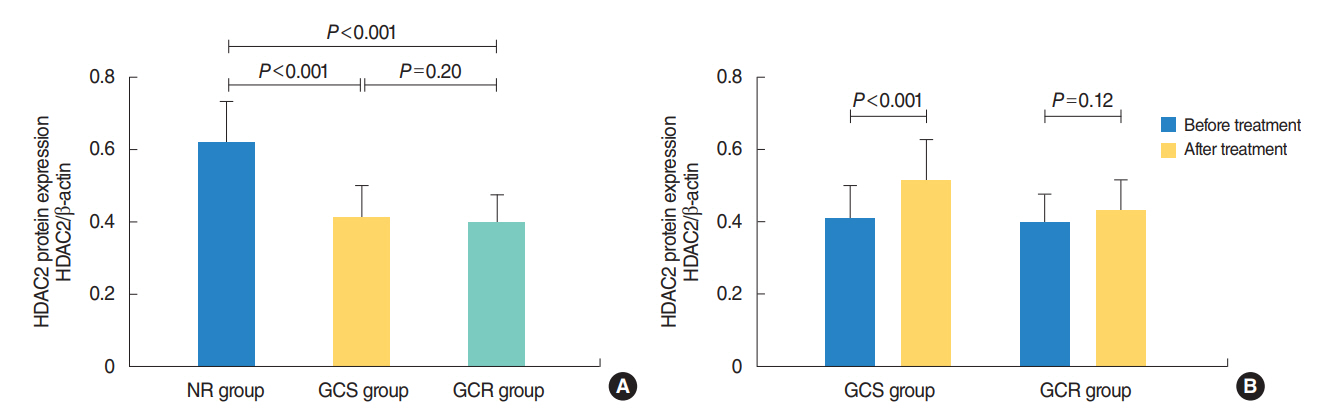
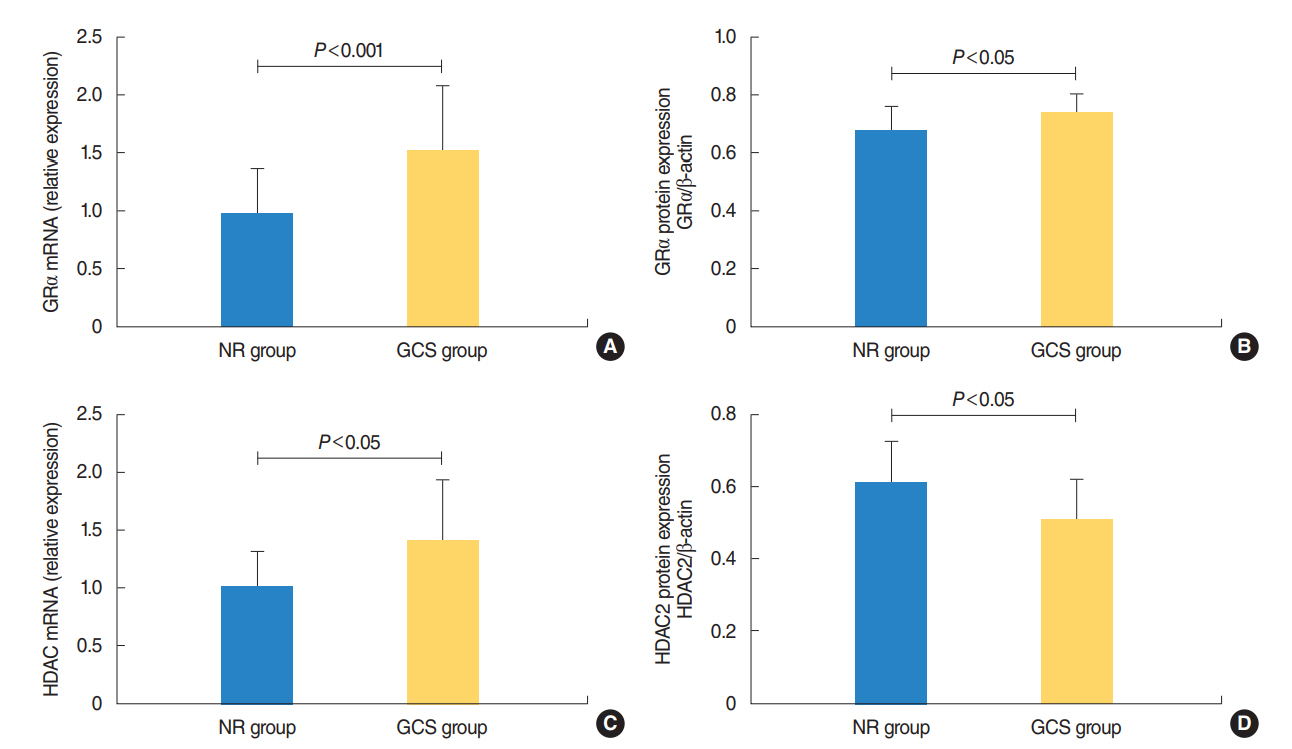
The protein levels of GRs and HDAC2 in PBMCs of SSNHL patients were lower than the normal reference values before GC treatment. Compared with the GC-resistant group, both the mRNA and protein levels of GRα and HDAC2 were significantly increased in the GC-sensitive group after GC treatment.
CONCLUSION
A lack of GRα and HDAC2 induction following steroid treatment in GC-resistant SSNHL patients may play a fundamental mechanistic role in GC insensitivity. Response of GRα and HDAC2 to steroid treatment may, thus, predict the prognosis of hearing improvement in SSNHL patients.
MeSH Terms
-
Blotting, Western
Gene Expression
Healthy Volunteers
Hearing
Hearing Loss
Hearing Loss, Sensorineural*
Histone Deacetylase 2*
Histone Deacetylases*
Histones*
Humans
Prognosis*
Real-Time Polymerase Chain Reaction
Receptors, Glucocorticoid*
Reference Values
RNA, Messenger
Histone Deacetylase 2
Histone Deacetylases
Histones
RNA, Messenger
Receptors, Glucocorticoid
Figure
Reference
-
1. El Sabbagh NG, Sewitch MJ, Bezdjian A, Daniel SJ. Intratympanic dexamethasone in sudden sensorineural hearing loss: a systematic review and meta-analysis. Laryngoscope. 2017; Aug. 127(8):1897–908.
Article2. Trune DR, Canlon B. Corticosteroid therapy for hearing and balance disorders. Anat Rec (Hoboken). 2012; Nov. 295(11):1928–43.3. Stachler RJ, Chandrasekhar SS, Archer SM, Rosenfeld RM, Schwartz SR, Barrs DM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012; Mar. 146(3 Suppl):S1–35.
Article4. Michel O; Deutsche Gesellschaft fur Hals-Nasen-Ohren-Heilkunde; Kopf- und Hals-Chirurgie. The revised version of the German guidelines “sudden idiopathic sensorineural hearing loss”. Laryngorhinootologie. 2011; May. 90(5):290–3.5. Gul F, Muderris T, Yalciner G, Sevil E, Bercin S, Ergin M, et al. A comprehensive study of oxidative stress in sudden hearing loss. Eur Arch Otorhinolaryngol. 2017; Mar. 274(3):1301–8.
Article6. Niu N, Manickam V, Kalari KR, Moon I, Pelleymounter LL, Eckloff BW, et al. Human glucocorticoid receptor alpha gene (NR3C1) pharmacogenomics: gene resequencing and functional genomics. J Clin Endocrinol Metab. 2009; Aug. 94(8):3072–84.7. Pujols L, Mullol J, Roca-Ferrer J, Torrego A, Xaubet A, Cidlowski JA, et al. Expression of glucocorticoid receptor alpha- and beta-isoforms in human cells and tissues. Am J Physiol Cell Physiol. 2002; Oct. 283(4):C1324–31.8. Rarey KE, Curtis LM. Receptors for glucocorticoids in the human inner ear. Otolaryngol Head Neck Surg. 1996; Jul. 115(1):38–41.
Article9. She W, Dai Y, Du X, Yu C, Chen F, Wang J, et al. Hearing evaluation of intratympanic methylprednisolone perfusion for refractory sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2010; Feb. 142(2):266–71.
Article10. Yang N, Ray DW, Matthews LC. Current concepts in glucocorticoid resistance. Steroids. 2012; Sep. 77(11):1041–9.
Article11. Li LB, Leung DY, Martin RJ, Goleva E. Inhibition of histone deacetylase 2 expression by elevated glucocorticoid receptor beta in steroid-resistant asthma. Am J Respir Crit Care Med. 2010; Oct. 182(7):877–83.12. Butler CA, McQuaid S, Taggart CC, Weldon S, Carter R, Skibinski G, et al. Glucocorticoid receptor β and histone deacetylase 1 and 2 expression in the airways of severe asthma. Thorax. 2012; May. 67(5):392–8.
Article13. Ma L, Fang M, Liang Y, Xiang Y, Jia Z, Sun X, et al. Low expression of glucocorticoid receptor alpha isoform in adult immune thrombocytopenia correlates with glucocorticoid resistance. Ann Hematol. 2013; Jul. 92(7):953–60.
Article14. Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, et al. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med. 2006; Jan. 203(1):7–13.15. Hou J, She W, Du X, Dai Y, Xie L, Zhou Q. Histone Deacetylase 2 in sudden sensorineural hearing loss patients in response to intratympanic methylprednisolone perfusion. Otolaryngol Head Neck Surg. 2016; Jan. 154(1):164–70.
Article16. Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Guideline of diagnosis and treatment of sudden deafness (2015). Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015; Jun. 50(6):443–7.17. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; Dec. 25(4):402–8.18. Yu H, Li H. Association of vertigo with hearing outcomes in patients with sudden sensorineural hearing loss: a systematic review and meta- analysis. JAMA Otolaryngol Head Neck Surg. 2018; Aug. 144(8):677–83.19. Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013; Mar. 131(3):636–45.
Article20. Heinrich UR, Strieth S, Schmidtmann I, Stauber R, Helling K. Dexamethasone prevents hearing loss by restoring glucocorticoid receptor expression in the guinea pig cochlea. Laryngoscope. 2016; Jan. 126(1):E29–34.
Article21. Li J, Liu D, Wu J, Zhang D, Cheng B, Zhang Y, et al. Ginsenoside Rg1 attenuates ultraviolet B-induced glucocortisides resistance in keratinocytes via Nrf2/HDAC2 signalling. Sci Rep. 2016; Dec. 6:39336.
Article22. Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, et al. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006; Jul. 291(1):L46–57.23. Wilkinson L, Verhoog NJD, Louw A. Disease and treatment associated acquired glucocorticoid resistance. Endocr Connect. 2018; Dec. 7(12):R328–49.24. Mercado N, Thimmulappa R, Thomas CM, Fenwick PS, Chana KK, Donnelly LE, et al. Decreased histone deacetylase 2 impairs Nrf2 activation by oxidative stress. Biochem Biophys Res Commun. 2011; Mar. 406(2):292–8.
Article25. Hew M, Bhavsar P, Torrego A, Meah S, Khorasani N, Barnes PJ, et al. Relative corticosteroid insensitivity of peripheral blood mononuclear cells in severe asthma. Am J Respir Crit Care Med. 2006; Jul. 174(2):134–41.
Article26. Riml S, Schmidt S, Ausserlechner MJ, Geley S, Kofler R. Glucocorticoid receptor heterozygosity combined with lack of receptor auto-induction causes glucocorticoid resistance in Jurkat acute lymphoblastic leukemia cells. Cell Death Differ. 2004; Jul. 11 Suppl 1:S65–72.
Article27. Hausmann M, Herfarth H, Scholmerich J, Rogler G. Glucocorticoid receptor isoform expression does not predict steroid treatment response in IBD. Gut. 2007; Sep. 56(9):1328–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Dolichoectasia of Vertebrobasilar Artery Presenting Simultaneous Bilateral Sudden Sensorineural Hearing Loss with Vertigo
- Repression of TNF-alpha-induced IL-8 expression by the glucocorticoid receptor-beta involves inhibition of histone H4 acetylation
- Fatal Case of Klebsiella Meningitis Combined with Bilateral Sudden Sensorineural Hearing Loss: A Case Report and Literature Review
- The Characteristics and the Changes of Tinnitus according to the Recovery of Hearing Loss in the Patients with Sudden Hearing Loss
- Analysis of Prognosis in Patients with Sudden Sensorineural Hearing Loss and Dizziness