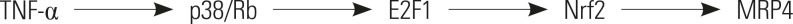Yonsei Med J.
2019 Nov;60(11):1045-1053. 10.3349/ymj.2019.60.11.1045.
TNFα Induces Multidrug Resistance-Associated Protein 4 Expression through p38-E2F1-Nrf2 Signaling in Obstructive Cholestasis
- Affiliations
-
- 1Department of Gastroenterology, Southwest Hospital, Army Medical University (Third Military Medical University), Chongqing, China. wenshengchenwsc@163.com
- KMID: 2460221
- DOI: http://doi.org/10.3349/ymj.2019.60.11.1045
Abstract
- PURPOSE
To explore the molecular mechanism of the upregulation of multidrug resistance-associated protein 4 (MRP4) in cholestasis.
MATERIALS AND METHODS
The mRNA and protein levels of MRP4 in liver samples from cholestatic patients were determined by quantitative real-time PCR and Western blot. In human hepatoma HepG2 cells, electrophoretic mobility shift assay (EMSA) was used to determine the affinity of nuclear factor-E2-related factor (Nrf2) binding to MRP4 promoter. Dual-luciferase reporter assay was used to detect the binding of tumor necrosis factor α (TNFα) to the promotor of E2F1. The bile duct ligation mouse models were established using male C57BL/6 mice.
RESULTS
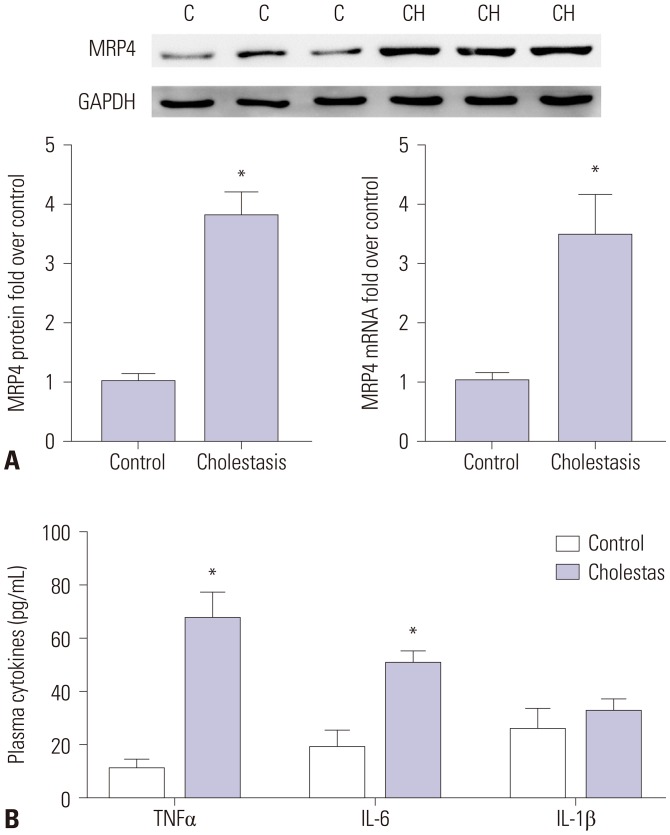
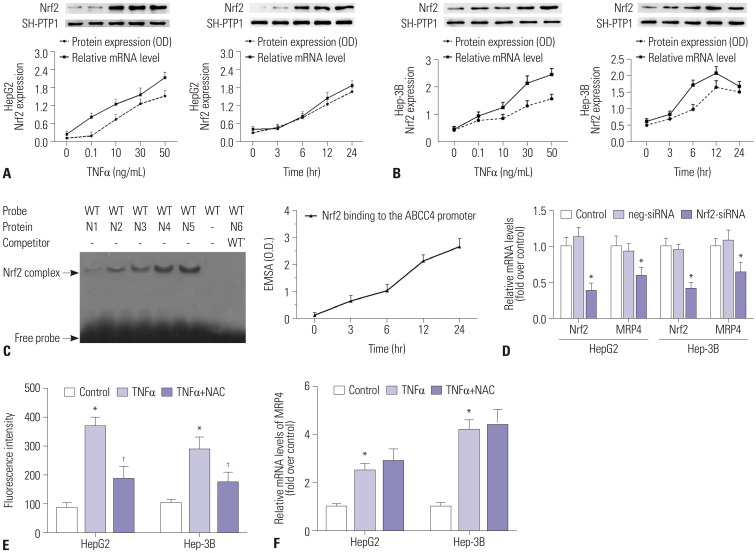
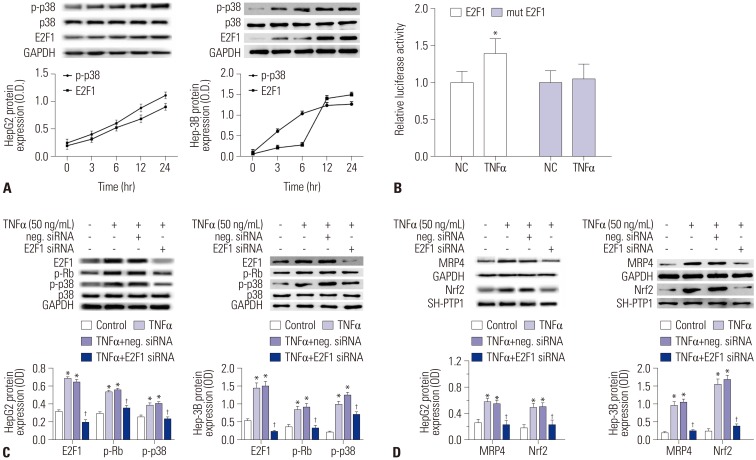
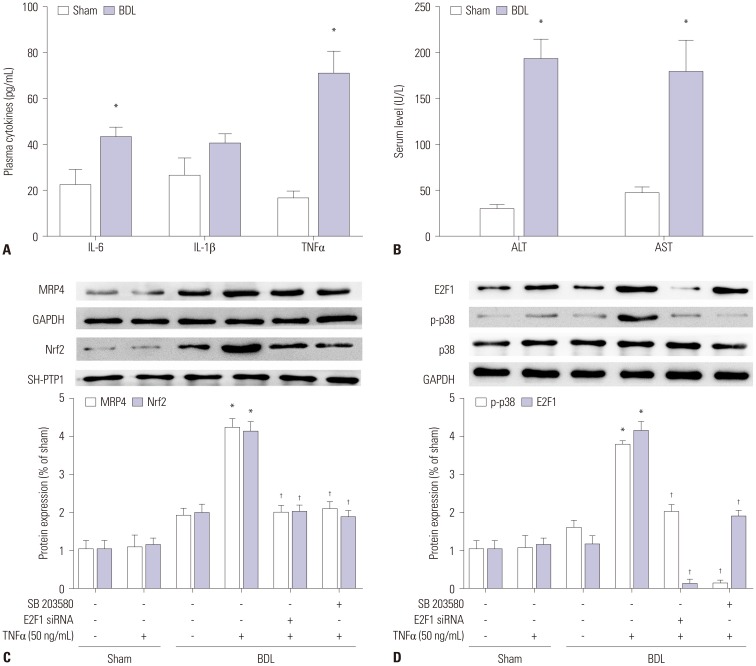
The mRNA and protein levels of MRP4 were significantly increased in cholestatic patients. TNFα treatment induced the expression of MRP4 and Nrf2 and enhanced cell nuclear extract binding activity to MRP4 promoter, as demonstrated by EMSA. Nrf2 knockdown reduced MRP4 mRNA levels in both HepG2 and Hep-3B cells. In addition, TNFα increased Rb phosphorylation and expression of MRP4 and Nrf2 and activated E2F1 and phosphorylated p38 in HepG2 and Hep-3B cells. These effects were markedly inhibited by pretreatment with E2F1 siRNA. Dual-luciferase reporter assay validated that TNFα induces the transcription of E2F1. Furthermore, the expression of MRP4, Nrf2, E2F1, and p-p38 proteins was improved with treatment of TNFα in a mouse model of cholestasis. E2F1 siRNA lentivirus or SB 203580 (p38 inhibitor) inhibited these positive effects.
CONCLUSION
Our findings indicated that TNFα induces hepatic MRP4 expression through activation of the p38-E2F1-Nrf2 signaling pathway in human obstructive cholestasis.
MeSH Terms
-
Animals
Bile Ducts
Blotting, Western
Carcinoma, Hepatocellular
Cholestasis*
Electrophoretic Mobility Shift Assay
Hep G2 Cells
Humans
Lentivirus
Ligation
Liver
Male
Mice
Multidrug Resistance-Associated Proteins*
Phosphorylation
Real-Time Polymerase Chain Reaction
RNA, Messenger
RNA, Small Interfering
Tumor Necrosis Factor-alpha
Up-Regulation
Multidrug Resistance-Associated Proteins
RNA, Messenger
RNA, Small Interfering
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Miszczuk GS, Banales JM, Zucchetti AE, Pisani GB, Boaglio AC, Saez E, et al. Adaptive downregulation of Cl-/HCO3- exchange activity in rat hepatocytes under experimental obstructive cholestasis. PLoS One. 2019; 14:e0212215. PMID: 30789925.
Article2. Wagner M, Trauner M. Transcriptional regulation of hepatobiliary transport systems in health and disease: implications for a rationale approach to the treatment of intrahepatic cholestasis. Ann Hepatol. 2005; 4:77–99. PMID: 16010241.
Article3. Zollner G, Marschall HU, Wagner M, Trauner M. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations. Mol Pharm. 2006; 3:231–251. PMID: 16749856.
Article4. Wagner M, Zollner G, Trauner M. New molecular insights into the mechanisms of cholestasis. J Hepatol. 2009; 51:565–580. PMID: 19595470.
Article5. Schuetz JD, Connelly MC, Sun D, Paibir SG, Flynn PM, Srinivas RV, et al. MRP4: a previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med. 1999; 5:1048–1051. PMID: 10470083.
Article6. Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002; 71:537–592. PMID: 12045106.
Article7. Wielinga PR, van der Heijden I, Reid G, Beijnen JH, Wijnholds J, Borst P. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem. 2003; 278:17664–17671. PMID: 12637526.
Article8. Schuetz EG, Strom S, Yasuda K, Lecureur V, Assem M, Brimer C, et al. Disrupted bile acid homeostasis reveals an unexpected interaction among nuclear hormone receptors, transporters, and cytochrome P450. J Biol Chem. 2001; 276:39411–39418. PMID: 11509573.
Article9. Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, et al. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 2003; 125:825–838. PMID: 12949728.
Article10. Donner MG, Schumacher S, Warskulat U, Heinemann J, Häussinger D. Obstructive cholestasis induces TNF-alpha- and IL-1 -mediated periportal downregulation of Bsep and zonal regulation of Ntcp, Oatp1a4, and Oatp1b2. Am J Physiol Gastrointest Liver Physiol. 2007; 293:G1134–G1146. PMID: 17916651.11. Stedman CA, Liddle C, Coulter SA, Sonoda J, Alvarez JG, Moore DD, et al. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci U S A. 2005; 102:2063–2068. PMID: 15684063.
Article12. Petrick JS, Klaassen CD. Importance of hepatic induction of constitutive androstane receptor and other transcription factors that regulate xenobiotic metabolism and transport. Drug Metab Dispos. 2007; 35:1806–1815. PMID: 17627975.
Article13. Chen P, Zeng H, Wang Y, Fan X, Xu C, Deng R, et al. Low dose of oleanolic acid protects against lithocholic acid-induced cholestasis in mice: potential involvement of nuclear factor-E2-related factor 2-mediated upregulation of multidrug resistance-associated proteins. Drug Metab Dispos. 2014; 42:844–852. PMID: 24510383.
Article14. Bohan A, Chen WS, Denson LA, Held MA, Boyer JL. Tumor necrosis factor alpha-dependent up-regulation of Lrh-1 and Mrp3 (Abcc3) reduces liver injury in obstructive cholestasis. J Biol Chem. 2003; 278:36688–36698. PMID: 12837754.15. Chai J, He Y, Cai SY, Jiang Z, Wang H, Li Q, et al. Elevated hepatic multidrug resistance-associated protein 3/ATP-binding cassette subfamily C 3 expression in human obstructive cholestasis is mediated through tumor necrosis factor alpha and c-Jun NH2-terminal kinase/stress-activated protein kinase-signaling pathway. Hepatology. 2012; 55:1485–1494. PMID: 22105759.16. Zhang FX, Kirschning CJ, Mancinelli R, Xu XP, Jin Y, Faure E, et al. Bacterial lipopolysaccharide activates nuclear factor-kappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999; 274:7611–7614. PMID: 10075645.17. Lee J, Azzaroli F, Wang L, Soroka CJ, Gigliozzi A, Setchell KD, et al. Adaptive regulation of bile salt transporters in kidney and liver in obstructive cholestasis in the rat. Gastroenterology. 2001; 121:1473–1484. PMID: 11729126.
Article18. Chai J, Luo D, Wu X, Wang H, He Y, Li Q, et al. Changes of organic anion transporter MRP4 and related nuclear receptors in human obstructive cholestasis. J Gastrointest Surg. 2011; 15:996–1004. PMID: 21359593.
Article19. Renga B, Migliorati M, Mencarelli A, Cipriani S, D'Amore C, Distrutti E, et al. Farnesoid X receptor suppresses constitutive androstane receptor activity at the multidrug resistance protein-4 promoter. Biochim Biophys Acta. 2011; 1809:157–165. PMID: 21296199.
Article20. Chai J, Cai SY, Liu X, Lian W, Chen S, Zhang L, et al. Canalicular membrane MRP2/ABCC2 internalization is determined by Ezrin Thr567 phosphorylation in human obstructive cholestasis. J Hepatol. 2015; 63:1440–1448. PMID: 26212029.
Article21. Denk GU, Soroka CJ, Takeyama Y, Chen WS, Schuetz JD, Boyer JL. Multidrug resistance-associated protein 4 is up-regulated in liver but down-regulated in kidney in obstructive cholestasis in the rat. J Hepatol. 2004; 40:585–591. PMID: 15030973.
Article22. Geier A, Wagner M, Dietrich CG, Trauner M. Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration. Biochim Biophys Acta. 2007; 1773:283–308. PMID: 17291602.
Article23. Osawa Y, Hoshi M, Yasuda I, Saibara T, Moriwaki H, Kozawa O. Tumor necrosis factor-α promotes cholestasis-induced liver fibrosis in the mouse through tissue inhibitor of metalloproteinase-1 production in hepatic stellate cells. PLoS One. 2013; 8:e65251. PMID: 23755201.
Article24. Nagaki M, Naiki T, Brenner DA, Osawa Y, Imose M, Hayashi H, et al. Tumor necrosis factor alpha prevents tumor necrosis factor receptor-mediated mouse hepatocyte apoptosis, but not fas-mediated apoptosis: role of nuclear factor-kappaB. Hepatology. 2000; 32:1272–1279. PMID: 11093734.25. Malekshah OM, Lage H, Bahrami AR, Afshari JT, Behravan J. PXR and NF-κB correlate with the inducing effects of IL-1β and TNF-α on ABCG2 expression in breast cancer cell lines. Eur J Pharm Sci. 2012; 47:474–480. PMID: 22750628.
Article26. Eba S, Hoshikawa Y, Moriguchi T, Mitsuishi Y, Satoh H, Ishida K, et al. The nuclear factor erythroid 2-related factor 2 activator oltipraz attenuates chronic hypoxia-induced cardiopulmonary alterations in mice. Am J Respir Cell Mol Biol. 2013; 49:324–333. PMID: 23590302.
Article27. Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol. 2002; 3:11–20. PMID: 11823794.
Article28. Wang S, Nath N, Minden A, Chellappan S. Regulation of Rb and E2F by signal transduction cascades: divergent effects of JNK1 and p38 kinases. EMBO J. 1999; 18:1559–1570. PMID: 10075927.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- AITC induces MRP1 expression by protecting against CS/CSE-mediated DJ-1 protein degradation via activation of the DJ-1/Nrf2 axis
- Metformin alleviates chronic obstructive pulmonary disease and cigarette smoke extract-induced glucocorticoid resistance by activating the nuclear factor E2-related factor 2/heme oxygenase-1 signaling pathway
- IL-1beta Induces Lysozyme Overexpression through a Mechanism Involving ERK/p38 Mitogen Activated Protein Kinase Activation in Human Airway Epithelial Cells
- Isoegomaketone Upregulates Heme Oxygenase-1 in RAW264.7 Cells via ROS/p38 MAPK/Nrf2 Pathway
- Lonchocarpine Increases Nrf2/ARE-Mediated Antioxidant Enzyme Expression by Modulating AMPK and MAPK Signaling in Brain Astrocytes