Anat Cell Biol.
2019 Sep;52(3):286-295. 10.5115/acb.18.122.
Effect of melatonin on the onset of puberty in male juvenile rats
- Affiliations
-
- 1Department of Anatomy, Maheshwara Medical College and Hospital, Hyderabad, India. satyaprasad33@yahoo.co.in
- KMID: 2459544
- DOI: http://doi.org/10.5115/acb.18.122
Abstract
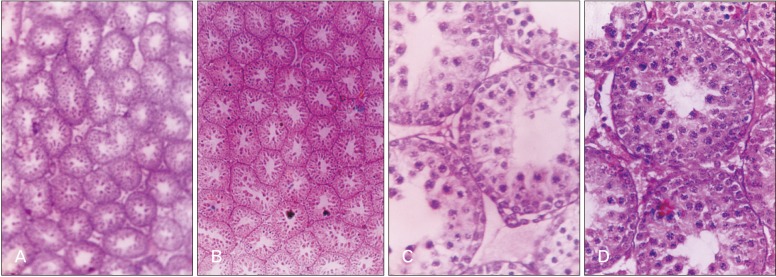
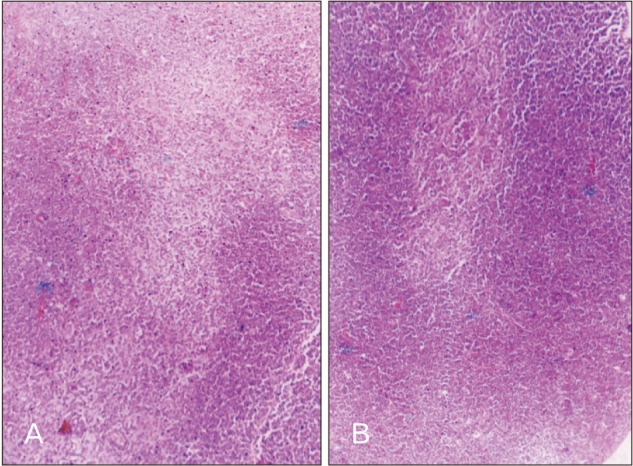
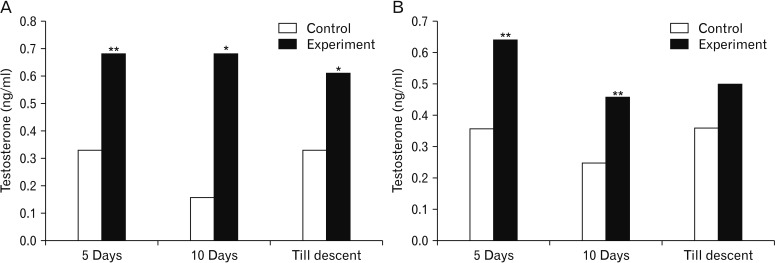
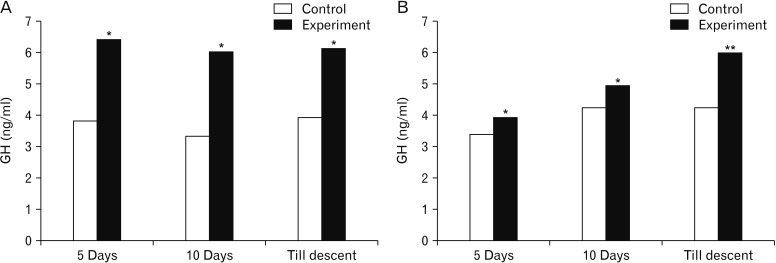
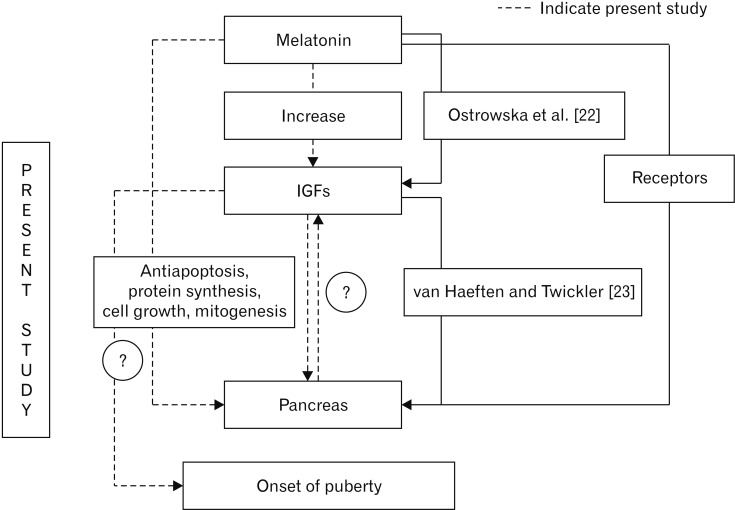
- Melatonin or N-acetyl-5-methoxytryptamine, the fascinating molecule secreted by the pineal gland. Melatonin has a close interaction with hypothalamic-pituitary-gonadal axis. In non-seasonal breeders like rat its exact role in reproduction is controvertible. So it is worth to explore the possible role of melatonin on the onset of puberty in male albino rats. Two groups of male rats aged 5 and 10 days were used for the study. In each group, there were three subgroups, each receiving melatonin for 5 days, 10 days or till the day of descent of testes. Similar subgroups were used as controls. Without handling, animals were observed daily for the onset of puberty. On the day of descent of testes, body weight of the animal was noted, blood was collected, serum was separated and used for radio immunoassay. For histomorphometric analysis, all morphometric measurements were done using an occular micrometer. Volume fraction of seminiferous tubules, intertubular connective tissue of testes, cortex and medulla of thymus were estimated by point count method. In both the age groups melatonin advanced the age on descent of testes, increased the body weight, organ weight. It also increased the serum hormone levels. So, in conclusion this study indicates that exogenous melatonin advances the onset of puberty in male albino wistar rats and this effect is more pronounced in the younger animals.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Alterations of Kiss 1 receptor, GnRH receptor and nuclear receptors of the hypothalamo-pituitary-ovarian axis following low dose bisphenol-A exposure in Wistar rats
Eniola Risikat Kadir, Aminu Imam, Olayemi Joseph Olajide, Moyosore Saliu Ajao
Anat Cell Biol. 2021;54(2):212-224. doi: 10.5115/acb.20.215.
Reference
-
1. Lerner AB, Case JD, Takahashi Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J Biol Chem. 1960; 235:1992–1997. PMID: 14415935.2. Das UN. Melatonin in human health and disease. J Assoc Phys India. 1996; 45:133–139.3. Weaver DR. The roles of melatonin in development. In : Olcese J, editor. Melatonin after Four Decades. New York: Kluwer Academic/Plenum Publishers;2000. p. 199–214.4. Minneman KP, Wurtman RJ. Effects of pineal compounds on mammals. Life Sci. 1975; 17:1189–1199. PMID: 1105043.5. Lang U, Aubert ML, Conne BS, Bradtke JC, Sizonenko PC. Influence of exogenous melatonin on melatonin secretion and the neuroendocrine reproductive axis of intact male rats during sexual maturation. Endocrinology. 1983; 112:1578–1584. PMID: 6299701.6. Mandal H, Ghosh PK, Biswas NM. Effect of dihydrotestosterone on serum concentrations of alpha 2u-globulin and on spermatogenesis in melatonin-treated rats. J Endocrinol. 1990; 126:431–435. PMID: 1698906.7. Pierpaoli W, Bulian D, Dall'Ara A, Marchetti B, Gallo F, Morale MC, Tirolo C, Testa N. Circadian melatonin and young-to-old pineal grafting postpone aging and maintain juvenile conditions of reproductive functions in mice and rats. Exp Gerontol. 1997; 32:587–602. PMID: 9315459.8. Alonso-Solís R, Abreu P, López-Coviella I, Hernández G, Fajardo N, Hernández-Díaz F, Díaz-Cruz A, Hernández A. Gonadal steroid modulation of neuroendocrine transduction: a transynaptic view. Cell Mol Neurobiol. 1996; 16:357–382. PMID: 8818402.9. Yilmaz B, Kutlu S, Mogulkoç R, Canpolat S, Sandal S, Tarakçi B, Kelestimur H. Melatonin inhibits testosterone secretion by acting at hypothalamo-pituitary-gonadal axis in the rat. Neuro Endocrinol Lett. 2000; 21:301–306. PMID: 11455362.10. Relkin R. Absence of alteration in puberal onset in male rats following amygdaloid lesioning. Endocrinology. 1971; 88:1272–1274. PMID: 5547247.11. Farris EJ, Griffith JQ Jr. The rat: in laboratory investigation. 2nd ed. Oxford: J.B.Lippincott Co.;1949.12. Drury RA, Wallington EA. Carleton's histological technique. 4th ed. New York: Oxford University Press;1967.13. Rat Behavior and Biology (Anne's rat page). Biological statistics of the Norway rat [Internet]. Rat Behavior and Biology;2003, 2004. cited 2019 Jan 1. Available from: http://www.ratbehavior.org/Stats.htm#RatStats.14. Moreno ML, Villanua MA, Esquifino AI. Serum prolactin and luteinizing hormone levels and the activities of hypothalamic monoamine oxidase A and B and phenylethanolamine-N-methyl transferase are changed during sexual maturation in male rats treated neonatally with melatonin. J Pineal Res. 1992; 13:167–173. PMID: 1287192.15. Kennedy GC, Mitra J. Body weight and food intake as initiating factors for puberty in the rat. J Physiol. 1963; 166:408–418. PMID: 14031944.16. Rasmussen DD, Boldt BM, Wilkinson CW, Yellon SM, Matsumoto AM. Daily melatonin administration at middle age suppresses male rat visceral fat, plasma leptin, and plasma insulin to youthful levels. Endocrinology. 1999; 140:1009–1012. PMID: 9927336.17. Peschke D, Peschke E, Mess B. Circannual rhythm and increase of body weight and food intake in the young wistar rat following pinealectomy and ganglionectomy. Neuroendocrinol Lett. 1987; 9:321–327.18. Herradon PG, Razquin B, Zapata AG. Effects of early partial decapitation on the ontogenic development of chicken lymphoid organs. I. Thymus. Am J Anat. 1991; 191:57–66. PMID: 1648305.19. Oner H, Kus I, Oner J, Ogeturk M, Ozan E, Ayar A. Possible effects of melatonin on thymus gland after pinealectomy in rats. Neuro Endocrinol Lett. 2004; 25:115–118. PMID: 15159694.20. Leja-Szpak A, Jaworek J, Nawrot-Porabka K, Palonek M, Mitis-Musioł M, Dembiński A, Konturek SJ, Pawlik WW. Modulation of pancreatic enzyme secretion by melatonin and its precursor: L-tryptophan. Role of CCK and afferent nerves. J Physiol Pharmacol. 2004; 55 Suppl 2:33–46. PMID: 15608359.21. Jaworek J, Nawrot K, Konturek SJ, Leja-Szpak A, Thor P, Pawlik WW. Melatonin and its precursor, L-tryptophan: influence on pancreatic amylase secretion in vivo and in vitro. J Pineal Res. 2004; 36:155–164. PMID: 15009505.22. Ostrowska Z, Kos-Kudla B, Swietochowska E, Marek B, Kajdaniuk D, Ciesielska-Kopacz N. Influence of pinealectomy and long-term melatonin administration on GH-IGF-I axis function in male rats. Neuro Endocrinol Lett. 2001; 22:255–262. PMID: 11524633.23. van Haeften TW, Twickler TB. Insulin-like growth factors and pancreas beta cells. Eur J Clin Invest. 2004; 34:249–255. PMID: 15086355.24. Daftary SS, Gore AC. IGF-1 in the brain as a regulator of reproductive neuroendocrine function. Exp Biol Med (Maywood). 2005; 230:292–306. PMID: 15855296.25. Edmonds KE, Stetson MH. Photoperiod and melatonin affect testicular growth in the marsh rice rat (Oryzomys palustris). J Pineal Res. 1994; 17:86–93. PMID: 7869231.26. Mahmoud I, Salman SS, al-Khateeb A. Continuous darkness and continuous light induce structural changes in the rat thymus. J Anat. 1994; 185(Pt 1):143–149. PMID: 7559109.27. Vaněcek J. The melatonin receptors in rat ontogenesis. Neuroendocrinology. 1988; 48:201–203. PMID: 2851752.28. Olivares AN, Valladares LE, Bustos-Obregón E, Núñez SM. Testicular function of sexually immature rats chronically treated with melatonin. Arch Biol Med Exp (Santiago). 1989; 22:387–393. PMID: 2488537.29. Esquifino AI, Arce A, Villanúa MA, Cardinali DP. Development of 24-hour rhythms in serum prolactin and luteinizing hormone levels in rats neonatally administered melatonin. Chronobiol Int. 1998; 15:21–28. PMID: 9493711.30. Acuña-Castroviejo D, Fernández B, Castillo JL, del Aguila CM. Similarity between the effects of suprachiasmatic nuclei lesions and of pinealectomy on gonadotropin release in ovariectomized, sulpiride-treated and melatonin-replaced rats. Experientia. 1993; 49:797–801. PMID: 8405305.31. Tijmes M, Pedraza R, Valladares L. Melatonin in the rat testis: evidence for local synthesis. Steroids. 1996; 61:65–68. PMID: 8750434.32. Vera H, Tijmes M, Valladares LE. Melatonin and testicular function: characterization of binding sites for 2-[125I]-iodomelatonin in immature rat testes. Steroids. 1997; 62:226–229. PMID: 9055381.33. Jevdjovic T, Maake C, Zwimpfer C, Krey G, Eppler E, Zapf J, Reinecke M. The effect of hypophysectomy on pancreatic islet hormone and insulin-like growth factor I content and mRNA expression in rat. Histochem Cell Biol. 2005; 123:179–188. PMID: 15812646.34. Kostoglou-Athanassiou I, Treacher DF, Wheeler MJ, Forsling ML. Melatonin administration and pituitary hormone secretion. Clin Endocrinol (Oxf). 1998; 48:31–37. PMID: 9509065.35. Smythe GA, Lazarus L. Growth hormone responses to melatonin in man. Science. 1974; 184:1373–1374. PMID: 4833260.36. Valcavi R, Dieguez C, Azzarito C, Edwards CA, Dotti C, Page MD, Portioli I, Scanlon MF. Effect of oral administration of melatonin on GH responses to GRF 1-44 in normal subjects. Clin Endocrinol (Oxf). 1987; 26:453–458. PMID: 3115632.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antinociceptive Effects of Intrathecal Melatonin on Formalin: and Thermal-induced Pain in Rats
- The effect of melatonin on cardio fibrosis in juvenile rats with pressure overload and deregulation of HDACs
- Effect of Melatonin on the Diabetes Mellitus Induced by Streptozotocin in Rats
- Protective Effect of Melatonin on Neuropathy in Streptozotocin-Induced Diabetic Rats
- The Effect of Combination Treatment of Melatonin and Hypothermia on Hypoxic-Ischemic Brain Injury in Neonatal Rats