Korean J Physiol Pharmacol.
2018 Nov;22(6):607-616. 10.4196/kjpp.2018.22.6.607.
The effect of melatonin on cardio fibrosis in juvenile rats with pressure overload and deregulation of HDACs
- Affiliations
-
- 1Key Laboratory of Pediatrics in Chongqing, Chongqing 400014, P.R. China; Chongqing International Science and Technology Cooperation Center for Child Development and Disorders, Chongqing 400014, P.R. China. qjyi2003@hotmail.com
- 2Department of Cardiovascular Medicine, Children's Hospital of Chongqing Medical University, Chongqing 400014, P.R. China.
- KMID: 2430083
- DOI: http://doi.org/10.4196/kjpp.2018.22.6.607
Abstract
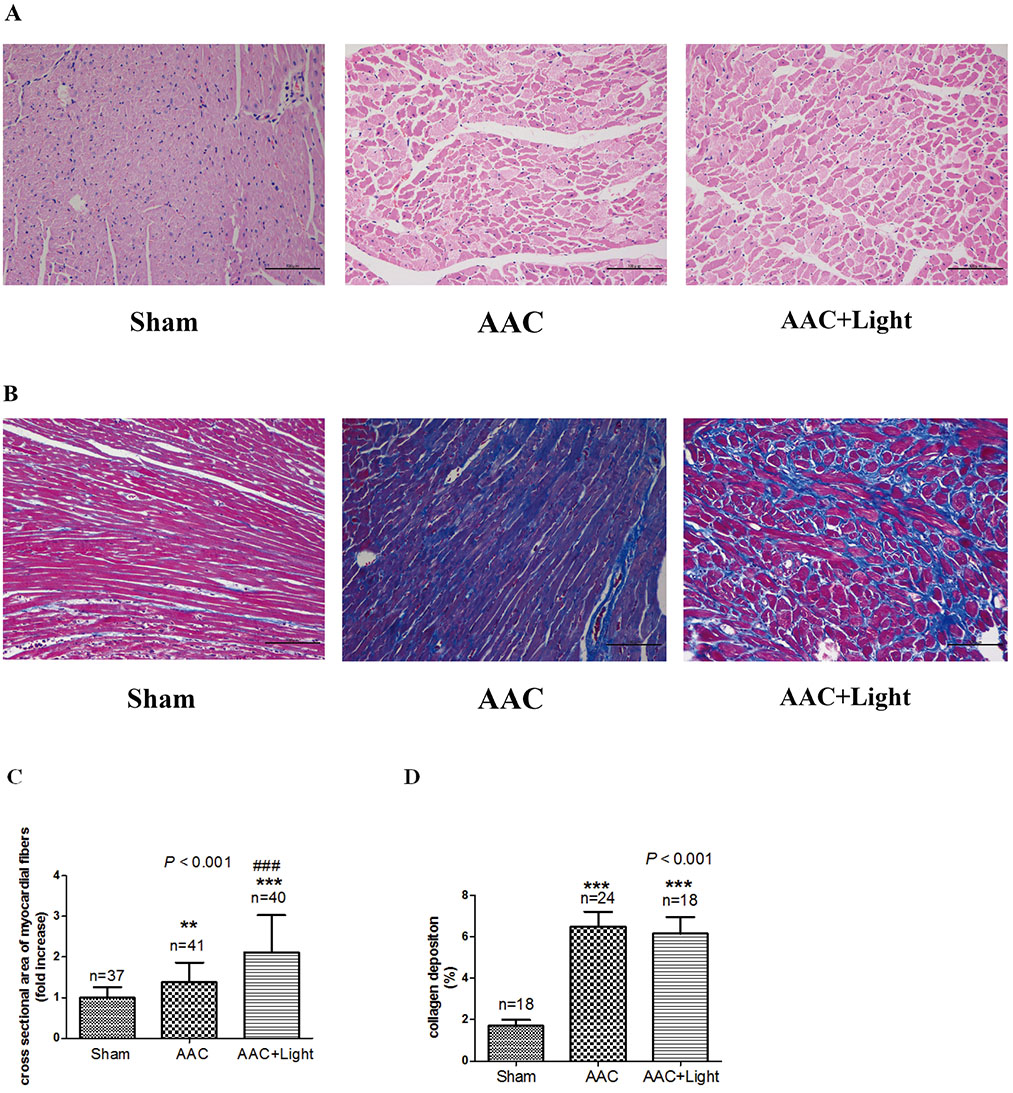
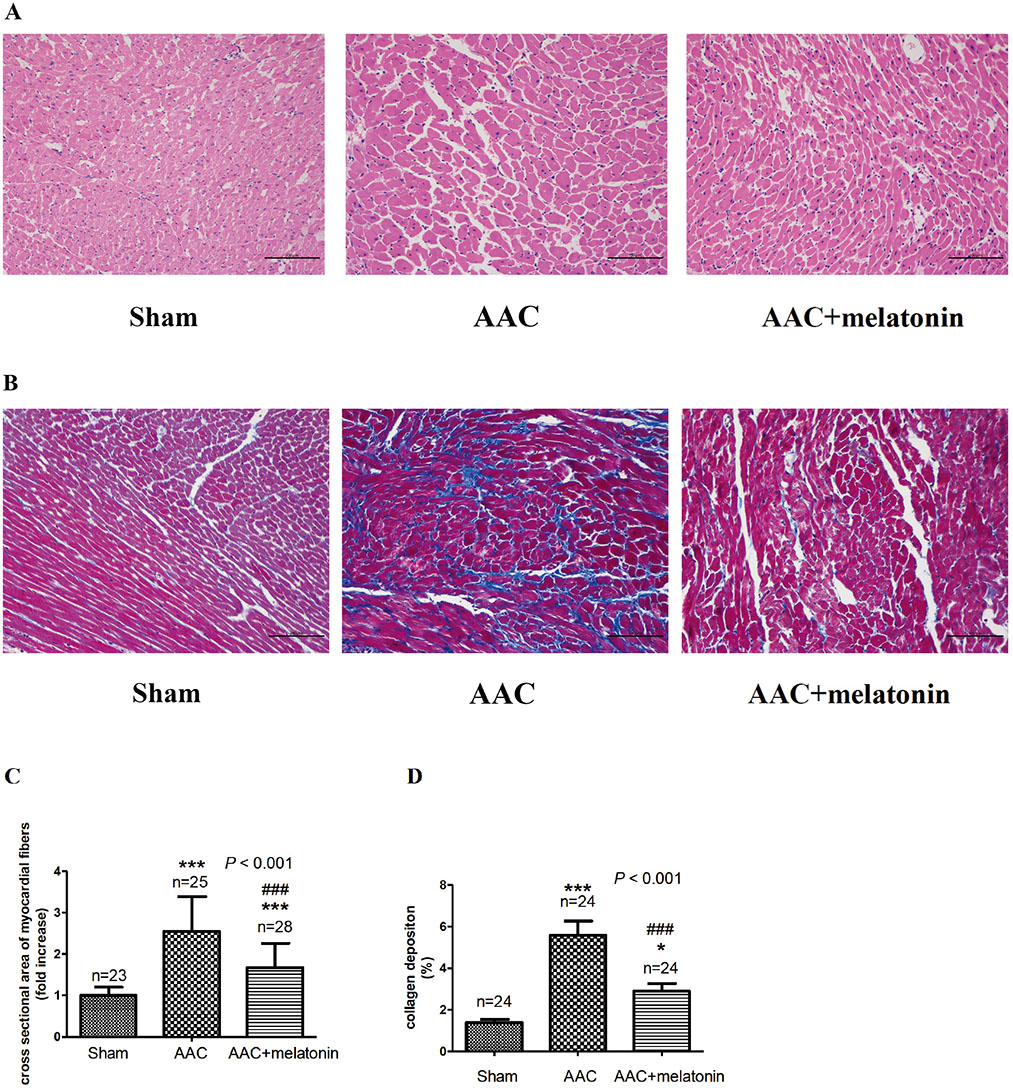
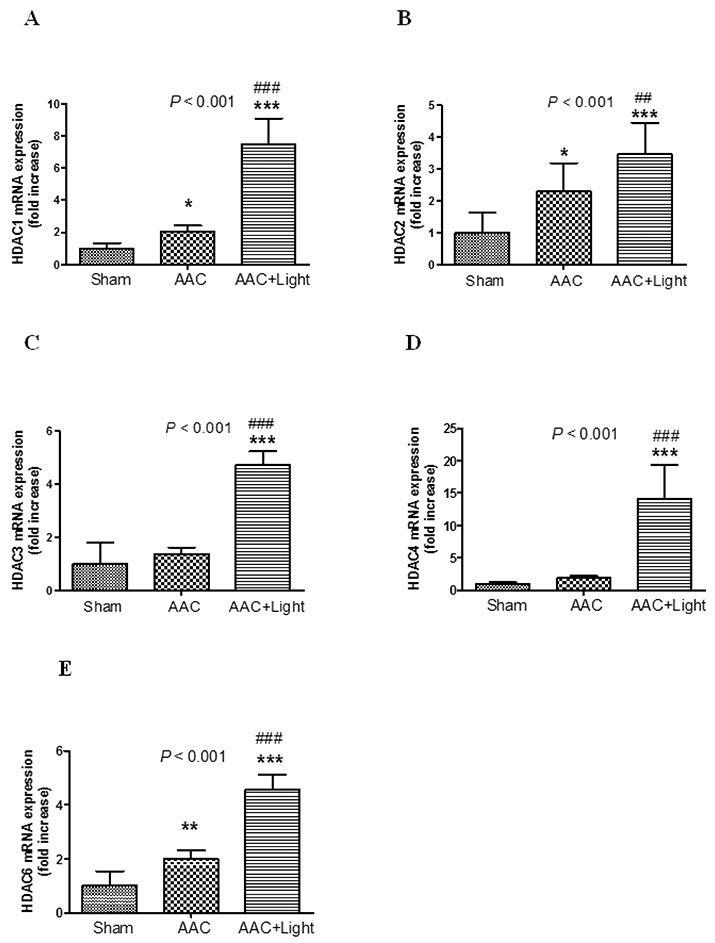
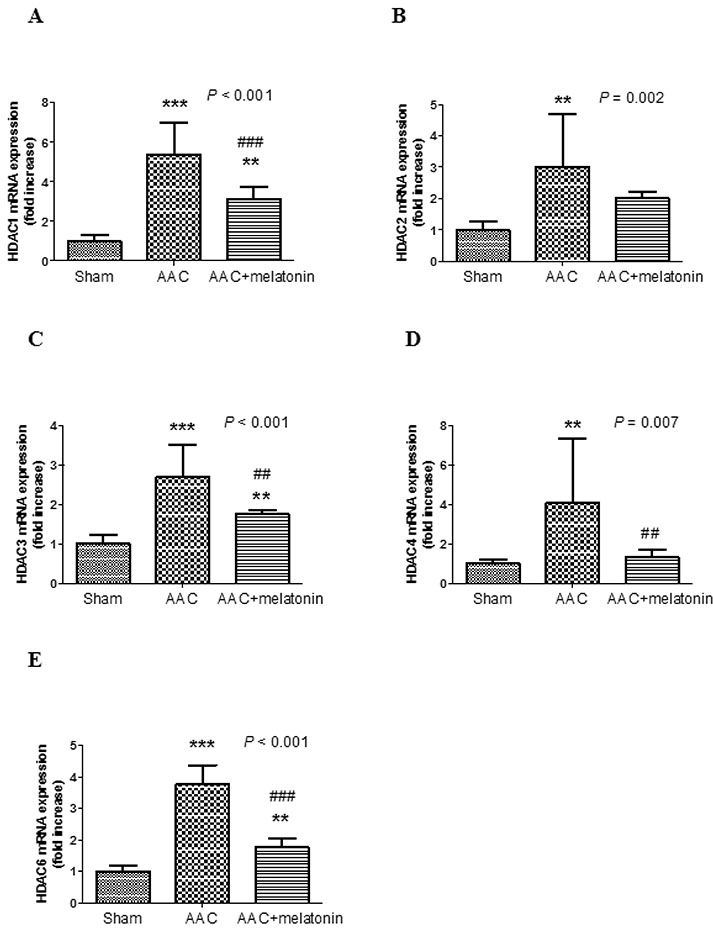
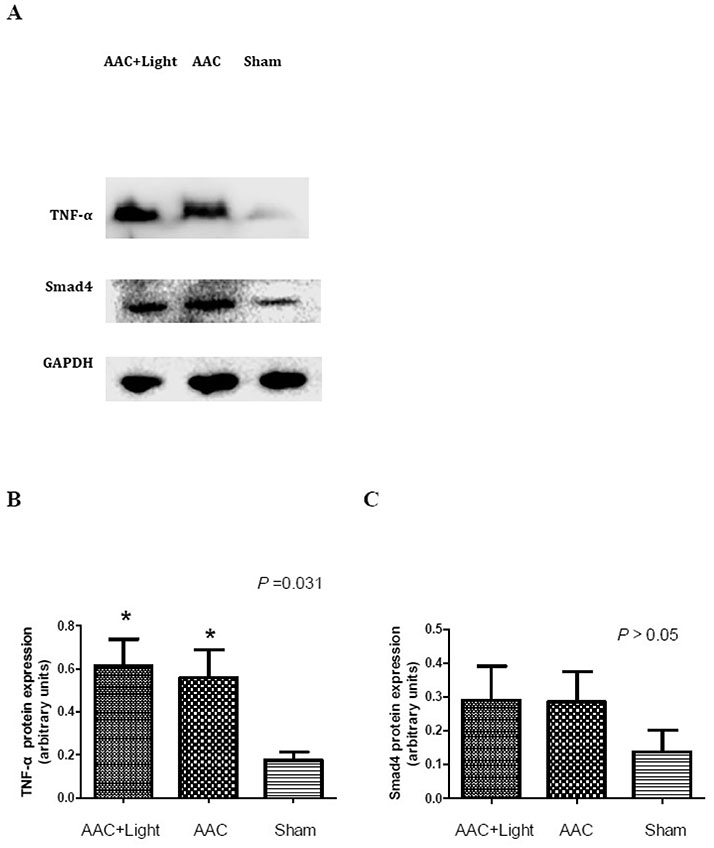
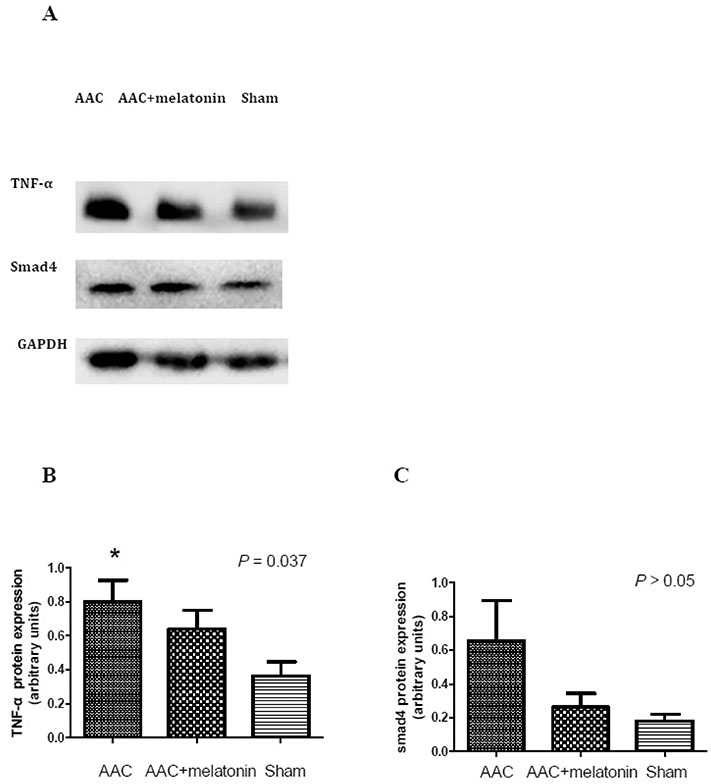
- The effect of melatonin on juveniles with cardio fibrosis is poorly understood. We investigated whether HDACs participate in the anti-fibrotic processes regulated by melatonin during hypertrophic remodeling. Abdominal aortic constriction (AAC) was employed in juvenile rats resulting in pressure overload-induced ventricular hypertrophy and melatonin was subsequently decreased via continuous light exposure for 5 weeks after surgery. AAC rats displayed an increased cross-sectional area of myocardial fibers and significantly elevated collagen deposition compared to sham-operated rats, as measured by HE and Masson Trichrome staining. Continuous light exposure following surgery exacerbated the increase in the cross-sectional area of myocardial fibers. The expression of HDAC1, HDAC2, HDAC3, HDAC4 and HDAC6 genes were all significantly enhanced in AAC rats with light exposure relative to the other rats. Moreover, the protein level of TNF-α was also upregulated in the AAC light exposure groups when compared with the sham. However, Smad4 protein expression was unchanged in the juveniles' hearts. In contrast, beginning 5 weeks after the operation, the AAC rats were treated with melatonin (10 mg/kg, intraperitoneal injection every evening) or vehicle 4 weeks, and sham rats were given vehicle. The changes in the histological measures of cardio fibrosis and the gene expressions of HDAC1, HDAC2, HDAC3, HDAC4 and HDAC6 were attenuated by melatonin administration. The results reveal that melatonin plays a role in the development of cardio fibrosis and the expression of HDAC1, HDAC2, HDAC3, HDAC4 and HDAC6 in cardiomyocytes.
Keyword
MeSH Terms
Figure
Reference
-
1. Reiter RJ, Melchiorri D, Sewerynek E, Poeggeler B, Barlow-Walden L, Chuang J, Ortiz GG, Acuña-Castroviejo D. A review of the evidence supporting melatonin's role as an antioxidant. J Pineal Res. 1995; 18:1–11.
Article2. Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002; 2:181–197.
Article3. Simko F, Pechanova O, Pelouch V, Krajcirovicova K, Celec P, Palffy R, Bednarova K, Vrankova S, Adamcova M, Paulis L. Continuous light and L-NAME-induced left ventricular remodelling: different protection with melatonin and captopril. J Hypertens. 2010; 28:Suppl 1. S13–S18.
Article4. Grossman E, Laudon M, Yalcin R, Zengil H, Peleg E, Sharabi Y, Kamari Y, Shen-Orr Z, Zisapel N. Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am J Med. 2006; 119:898–902.
Article5. Ferguson BS, McKinsey TA. Non-sirtuin histone deacetylases in the control of cardiac aging. J Mol Cell Cardiol. 2015; 83:14–20.
Article6. Schuetze KB, McKinsey TA, Long CS. Targeting cardiac fibroblasts to treat fibrosis of the heart: focus on HDACs. J Mol Cell Cardiol. 2014; 70:100–107.
Article7. Gallo P, Latronico MV, Gallo P, Grimaldi S, Borgia F, Todaro M, Jones P, Gallinari P, De Francesco R, Ciliberto G, Steinkühler C, Esposito G, Condorelli G. Inhibition of class I histone deacetylase with an apicidin derivative prevents cardiac hypertrophy and failure. Cardiovasc Res. 2008; 80:416–424.
Article8. Kang SH, Seok YM, Song MJ, Lee HA, Kurz T, Kim I. Histone deacetylase inhibition attenuates cardiac hypertrophy and fibrosis through acetylation of mineralocorticoid receptor in spontaneously hypertensive rats. Mol Pharmacol. 2015; 87:782–791.
Article9. Wu TH, Kuo HC, Lin IC, Chien SJ, Huang LT, Tain YL. Melatonin prevents neonatal dexamethasone induced programmed hypertension: histone deacetylase inhibition. J Steroid Biochem Mol Biol. 2014; 144:253–259.
Article10. Hwang B, Qu TY, Hu CT, Chen HI. Hemodynamic and neurohumoral changes after abdominal aortic constriction in rats. Proc Natl Sci Counc Repub China B. 1999; 23:149–157.11. Şehirli AÖ, Koyun D, Tetik Ş, Özsavcı D, Yiğiner Ö, Çetinel Ş, Tok OE, Kaya Z, Akkiprik M, Kılıç E, Şener G. Melatonin protects against ischemic heart failure in rats. J Pineal Res. 2013; 55:138–148.
Article12. Zhang S, Li H, Yang SJ. Tribulosin protects rat hearts from ischemia/reperfusion injury. Acta Pharmacol Sin. 2010; 31:671–678.
Article13. Kang S, Liu Y, Sun D, Zhou C, Liu A, Xu C, Hao Y, Li D, Yan C, Sun H. Chronic activation of the G protein-coupled receptor 30 with agonist G-1 attenuates heart failure. PLoS One. 2012; 7:e48185.
Article14. Williams SM, Golden-Mason L, Ferguson BS, Schuetze KB, Cavasin MA, Demos-Davies K, Yeager ME, Stenmark KR, McKinsey TA. Class I HDACs regulate angiotensin II-dependent cardiac fibrosis via fibroblasts and circulating fibrocytes. J Mol Cell Cardiol. 2014; 67:112–125.
Article15. Mizrak B, Parlakpinar H, Acet A, Turkoz Y. Effects of pinealectomy and exogenous melatonin on rat hearts. Acta Histochem. 2004; 106:29–36.16. Lee E, Song MJ, Lee HA, Kang SH, Kim M, Yang EK, Lee DY, Ro S, Cho JM, Kim I. Histone deacetylase inhibitor, CG200745, attenuates cardiac hypertrophy and fibrosis in DOCA-induced hypertensive rats. Korean J Physiol Pharmacol. 2016; 20:477–485.
Article17. Lochner A, Huisamen B, Nduhirabandi F. Cardioprotective effect of melatonin against ischaemia/reperfusion damage. Front Biosci (Elite Ed). 2013; 5:305–315.18. Lkhagva B, Lin YK, Kao YH, Chazo TF, Chung CC, Chen SA, Chen YJ. Novel histone deacetylase inhibitor modulates cardiac peroxisome proliferator-activated receptors and inflammatory cytokines in heart failure. Pharmacology. 2015; 96:184–191.
Article19. Hohl M, Wagner M, Reil JC, Müller SA, Tauchnitz M, Zimmer AM, Lehmann LH, Thiel G, Böhm M, Backs J, Maack C. HDAC4 controls histone methylation in response to elevated cardiac load. J Clin Invest. 2013; 123:1359–1370.
Article20. Sucharov CC, Dockstader K, McKinsey TA. YY1 protects cardiac myocytes from pathologic hypertrophy by interacting with HDAC5. Mol Biol Cell. 2008; 19:4141–4153.
Article21. Trivedi CM, Luo Y, Yin Z, Zhang M, Zhu W, Wang T, Floss T, Goettlicher M, Noppinger PR, Wurst W, Ferrari VA, Abrams CS, Gruber PJ, Epstein JA. Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3 beta activity. Nat Med. 2007; 13:324–331.22. Korkmaz A, Rosales-Corral S, Reiter RJ. Gene regulation by melatonin linked to epigenetic phenomena. Gene. 2012; 503:1–11.
Article23. Bradham WS, Bozkurt B, Gunasinghe H, Mann D, Spinale FG. Tumor necrosis factor-alpha and myocardial remodeling in progression of heart failure: a current perspective. Cardiovasc Res. 2002; 53:822–830.
Article24. Gupta S, Tripathi CD. Current status of TNF blocking therapy in heart failure. Indian J Med Sci. 2005; 59:363–366.
Article25. Shaki F, Ashari S, Ahangar N. Melatonin can attenuate ciprofloxacin induced nephrotoxicity: Involvement of nitric oxide and TNF-α. Biomed Pharmacother. 2016; 84:1172–1178.
Article26. Hao J, Ju H, Zhao S, Junaid A, Scammell-La Fleur T, Dixon IM. Elevation of expression of Smads 2, 3, and 4, decorin and TGF-beta in the chronic phase of myocardial infarct scar healing. J Mol Cell Cardiol. 1999; 31:667–678.27. Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF, Heller HC, Franken P, Bourgin P. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(-/-) mice. PLoS Biol. 2009; 7:e1000125.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of melatonin on the onset of puberty in male juvenile rats
- Protective Effect of Melatonin on Neuropathy in Streptozotocin-Induced Diabetic Rats
- The Effect of Combination Treatment of Melatonin and Hypothermia on Hypoxic-Ischemic Brain Injury in Neonatal Rats
- Antinociceptive Effects of Intrathecal Melatonin on Formalin: and Thermal-induced Pain in Rats
- Effect of Melatonin on the Diabetes Mellitus Induced by Streptozotocin in Rats