J Pathol Transl Med.
2019 Jul;53(4):217-224. 10.4132/jptm.2019.02.20.
Association between Expression of 8-OHdG and Cigarette Smoking in Non-small Cell Lung Cancer
- Affiliations
-
- 1Department of Pathology, Research Institute of Clinical Medicine of Chonbuk National University, Biomedical Research Institute of Chonbuk National University Hospital, Research Institute for Endocrine Sciences, Chonbuk National University Medical School, Jeonju, Korea. mjchung@jbnu.ac.kr
- 2Department of Internal Medicine, Research Institute of Clinical Medicine of Chonbuk National University, Biomedical Research Institute of Chonbuk National University Hospital, Research Institute for Endocrine Sciences, Chonbuk National University Medical School, Jeonju, Korea.
- 3Department of Thoracic and Cardiovascular Surgery, Research Institute of Clinical Medicine of Chonbuk National University, Biomedical Research Institute of Chonbuk National University Hospital, Research Institute for Endocrine Sciences, Chonbuk National University Medical School, Jeonju, Korea.
- 4Department of Pharmacology, Research Institute of Clinical Medicine of Chonbuk National University, Biomedical Research Institute of Chonbuk National University Hospital, Research Institute for Endocrine Sciences, Chonbuk National University Medical School, Jeonju, Korea.
- KMID: 2454601
- DOI: http://doi.org/10.4132/jptm.2019.02.20
Abstract
- BACKGROUND
Exposure to cigarette smoking (CS) is a major risk factor for the development of lung cancer. CS is known to cause oxidative DNA damage and mutation of tumor-related genes, and these factors are involved in carcinogenesis. 8-Hydroxydeoxyguanosine (8-OHdG) is considered to be a reliable biomarker for oxidative DNA damage. Increased levels of 8-OHdG are associated with a number of pathological conditions, including cancer. There are no reports on the expression of 8-OHdG by immunohistochemistry in non-small cell lung cancer (NSCLC).
METHODS
We investigated the expression of 8-OHdG and p53 in 203 NSCLC tissues using immunohistochemistry and correlated it with clinicopathological features including smoking.
RESULTS
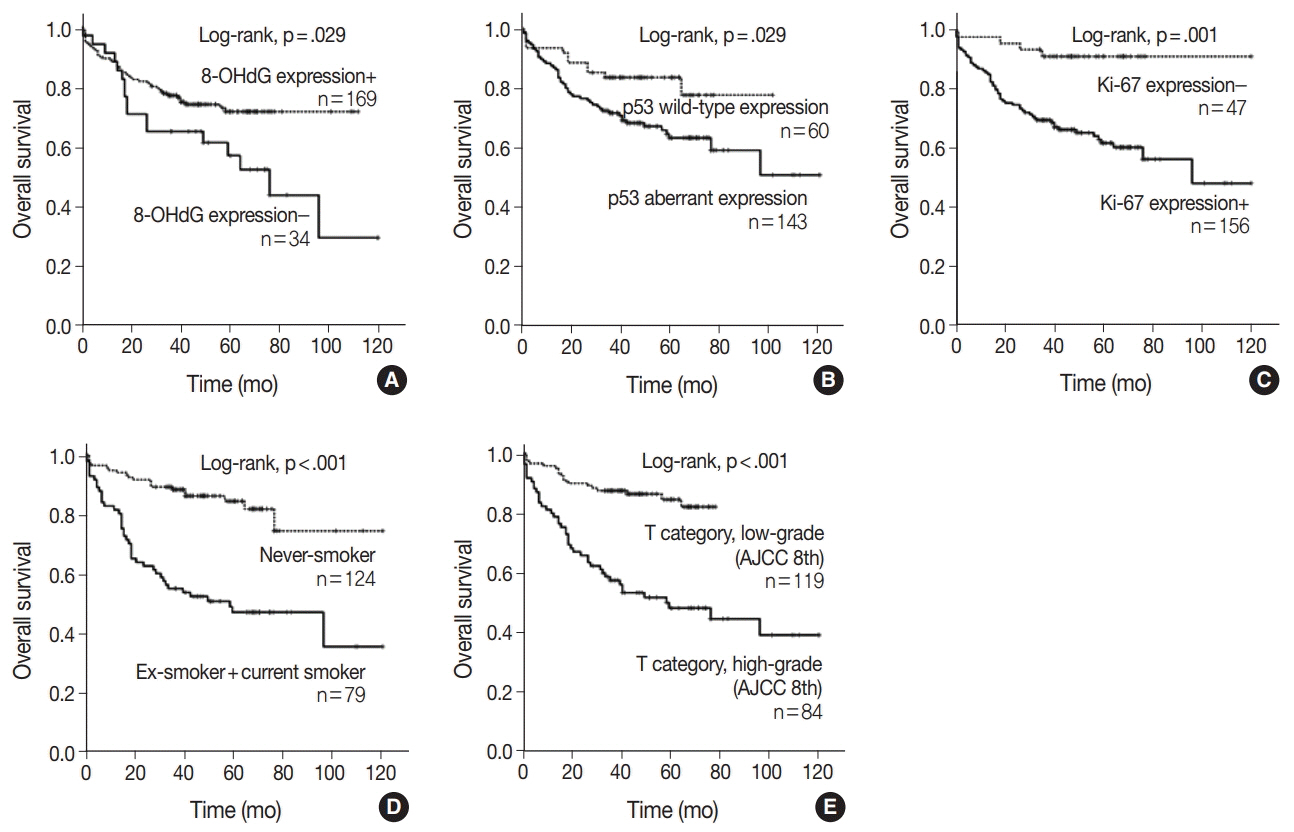
The expression of 8-OHdG was observed in 83.3% of NSCLC. It was significantly correlated with a low T category, negative lymph node status, never-smoker, and longer overall survival (p < .05) by univariate analysis. But multivariate analysis revealed that 8-OHdG was not an independent prognostic factor for overall survival in NSCLC patients. The aberrant expression of p53 significantly correlated with smoking, male, squamous cell carcinoma, and Ki-67 positivity (p < .05).
CONCLUSIONS
The expression of 8-OHdG was associated with good prognostic factors. It was positively correlated with never-smokers in NSCLC, suggesting that oxidative damage of DNA cannot be explained by smoking alone and may depend on complex control mechanisms.
Keyword
MeSH Terms
Figure
Reference
-
1. Ferlay J, Soerjomataram I, Ervik M, et al. Cancer incidence and mortality worldwide: IARC CancerBase No. 11. Lyon: International Agency for Research on Cancer;2014.2. David SS, O'Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007; 447:941–50.
Article3. Matsumoto K, Satoh Y, Sugo H, et al. Immunohistochemical study of the relationship between 8-hydroxy-2'-deoxyguanosine levels in noncancerous region and postoperative recurrence of hepatocellular carcinoma in remnant liver. Hepatol Res. 2003; 25:435–41.
Article4. Sova H, Jukkola-Vuorinen A, Puistola U, Kauppila S, Karihtala P. 8-Hydroxydeoxyguanosine: a new potential independent prognostic factor in breast cancer. Br J Cancer. 2010; 102:1018–23.
Article5. Gibbons DL, Byers LA, Kurie JM. Smoking, p53 mutation, and lung cancer. Mol Cancer Res. 2014; 12:3–13.
Article6. Murtas D, Piras F, Minerba L, et al. Nuclear 8-hydroxy-2'-deoxyguanosine as survival biomarker in patients with cutaneous melanoma. Oncol Rep. 2010; 23:329–35.
Article7. Rami-Porta R, Asamura H, Travis WD, Rusch VW. Lung. In: Amin MB, ed. AJCC cancer staging manual. Cham: Springer;2017. p. 431–56. 8th.8. Yemelyanova A, Vang R, Kshirsagar M, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011; 24:1248–53.
Article9. Cha YJ, Kim HR, Lee CY, Cho BC, Shim HS. Clinicopathological and prognostic significance of programmed cell death ligand-1 expression in lung adenocarcinoma and its relationship with p53 status. Lung Cancer. 2016; 97:73–80.
Article10. Shim HS, Kenudson M, Zheng Z, et al. Unique genetic and survival characteristics of invasive mucinous adenocarcinoma of the lung. J Thorac Oncol. 2015; 10:1156–62.
Article11. Shen J, Deininger P, Hunt JD, Zhao H. 8-Hydroxy-2'-deoxyguanosine (8-OH-dG) as a potential survival biomarker in patients with nonsmall-cell lung cancer. Cancer. 2007; 109:574–80.
Article12. Kaczmarek P, Blaszczyk J, Fijalkowski P, et al. Assessment of 8-hydroxy-2'-deoxyguanosine concentrations in bladder cancer patients treated with intravesical BCG instillation. Pol Merkur Lekarski. 2005; 19:526–8.13. Oliva MR, Ripoll F, Muniz P, et al. Genetic alterations and oxidative metabolism in sporadic colorectal tumors from a Spanish community. Mol Carcinog. 1997; 18:232–43.
Article14. Okamoto K, Toyokuni S, Uchida K, et al. Formation of 8-hydroxy-2'-deoxyguanosine and 4-hydroxy-2-nonenal-modified proteins in human renal-cell carcinoma. Int J Cancer. 1994; 58:825–9.
Article15. Miyake H, Hara I, Kamidono S, Eto H. Oxidative DNA damage in patients with prostate cancer and its response to treatment. J Urol. 2004; 171:1533–6.
Article16. Nagashima M, Tsuda H, Takenoshita S, et al. 8-hydroxydeoxyguanosine levels in DNA of human breast cancer are not significantly different from those of non-cancerous breast tissues by the HPLC-ECD method. Cancer Lett. 1995; 90:157–62.17. Jaloszynski P, Jaruga P, Olinski R, et al. Oxidative DNA base modifications and polycyclic aromatic hydrocarbon DNA adducts in squamous cell carcinoma of larynx. Free Radic Res. 2003; 37:231–40.18. Karihtala P, Kauppila S, Puistola U, Jukkola-Vuorinen A. Divergent behaviour of oxidative stress markers 8-hydroxydeoxyguanosine (8-OHdG) and 4-hydroxy-2-nonenal (HNE) in breast carcinogenesis. Histopathology. 2011; 58:854–62.
Article19. Toyokuni S, Tanaka T, Hattori Y, et al. Quantitative immunohistochemical determination of 8-hydroxy-2'-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab Invest. 1997; 76:365–74.20. Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993; 362:709–15.
Article21. Soini Y, Haapasaari KM, Vaarala MH, Turpeenniemi-Hujanen T, Kärjä V, Karihtala P. 8-hydroxydeguanosine and nitrotyrosine are prognostic factors in urinary bladder carcinoma. Int J Clin Exp Pathol. 2011; 4:267–75.22. Takahashi S, Hirose M, Tamano S, et al. Immunohistochemical detection of 8-hydroxy-2'-deoxyguanosine in paraffin-embedded sections of rat liver after carbon tetrachloride treatment. Toxicol Pathol. 1998; 26:247–52.
Article23. Valavanidis A, Vlachogianni T, Fiotakis K, Loridas S. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health. 2013; 10:3886–907.
Article24. Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004; 9 Suppl 5:4–9.
Article25. Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res. 1993; 73:1100–12.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- p 53 Expression in Non - Small Cell Lung Cancer: Its relationship to the clinical prognostic factor and smoking history
- The association between smoking and asthma
- Immunohistochemical Study on the Expression of p53 and Bcl-2 Proteins in Non-Small Cell Lung Carcinomas
- Gender Differences of Susceptibility to Lung Cancer According to Smoking Habits
- Immunohistochemical Study of the Expression of the p53 Protein in Primary Lung Cancer