Cancer Res Treat.
2019 Jan;51(1):357-367. 10.4143/crt.2017.457.
Risk Factor Analysis for Secondary Malignancy in Dexrazoxane-Treated Pediatric Cancer Patients
- Affiliations
-
- 1Department of Pediatrics, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. hyshin@snu.ac.kr
- 2Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 4Department of Pediatrics, Yeungnam University Medical Center, Yeungnam University College of Medicine, Daegu, Korea.
- 5Department of Pediatrics, Daegu Fatima Hospital, Daegu, Korea.
- 6Department of Pediatrics, Korean Cancer Center Hospital, Seoul, Korea.
- 7Department of Pediatrics, Hanyang University Medical Center, Hanyang University College of Medicine, Seoul, Korea.
- 8Department of Pediatrics, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea.
- 9Department of Pediatrics, Chonnam National University Hwasun Hospital, Chonnam National University School of Medicine, Hwasun, Korea.
- 10Department of Pediatrics, Keimyung University Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea.
- 11Department of Pediatrics, School of Medicine, Kyungpook National University, Daegu, Korea.
- 12Department of Pediatrics, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 13Department of Pediatrics, Dong-A University Hospital, Dong-A University College of Medicine, Busan, Korea.
- 14Department of Pediatrics, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea.
- 15Department of Pediatrics, Kyung Hee University Medical Center, Kyung Hee University School of Medicine, Seoul, Korea.
- 16Department of Pediatrics, Dankook University Hospital, Dankook University College of Medicine, Cheonan, Korea.
- 17Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea.
- 18Department of Pediatrics, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan.
- 19Medical Research Collaborating Center, Seoul National University Hospital, Seoul, Korea.
- KMID: 2437626
- DOI: http://doi.org/10.4143/crt.2017.457
Abstract
- PURPOSE
Dexrazoxane has been used as an effective cardioprotector against anthracycline cardiotoxicity. This study intended to analyze cardioprotective efficacy and secondary malignancy development, and elucidate risk factors for secondary malignancies in dexrazoxane-treated pediatric patients.
MATERIALS AND METHODS
Data was collected from 15 hospitals in Korea. Patients who received any anthracyclines, and completed treatment without stem cell transplantation were included. For efficacy evaluation, the incidence of cardiac events and cardiac event-free survival rates were compared. Data about risk factors of secondary malignancies were collected.
RESULTS
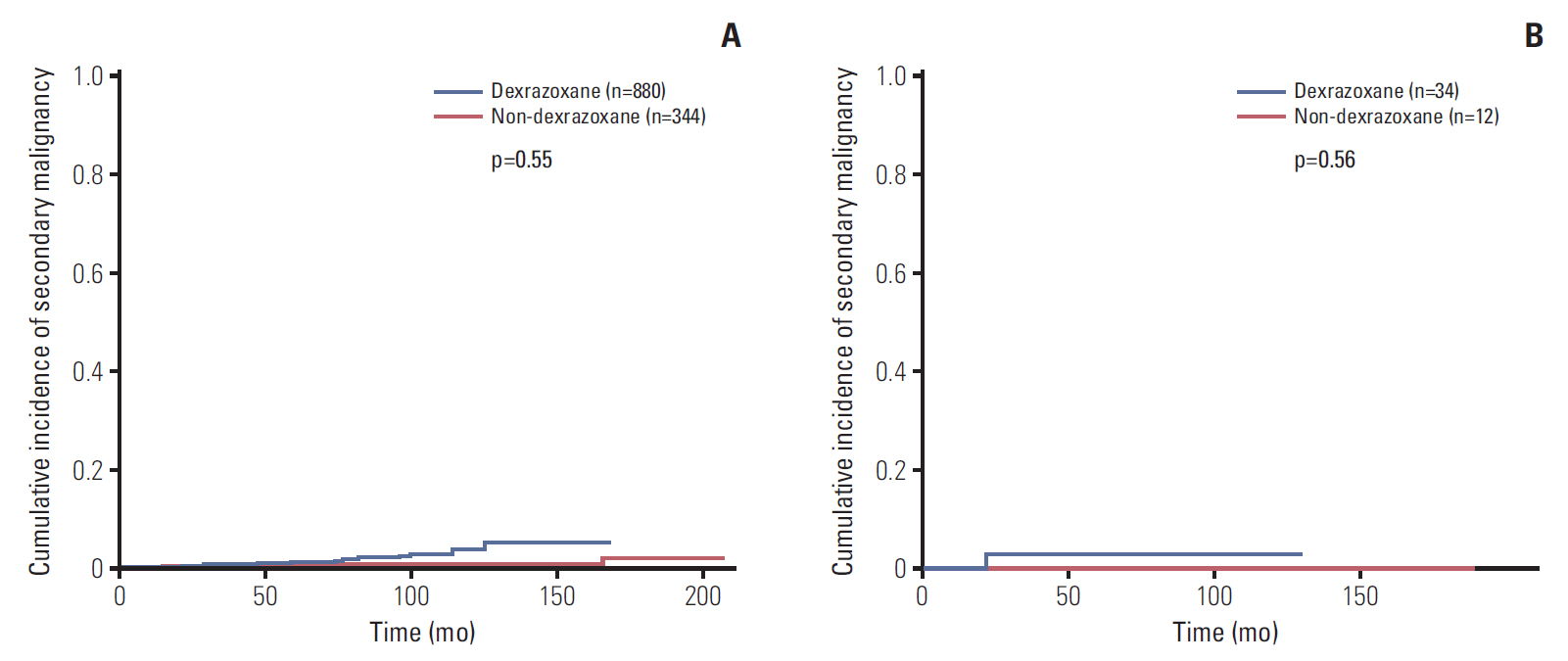
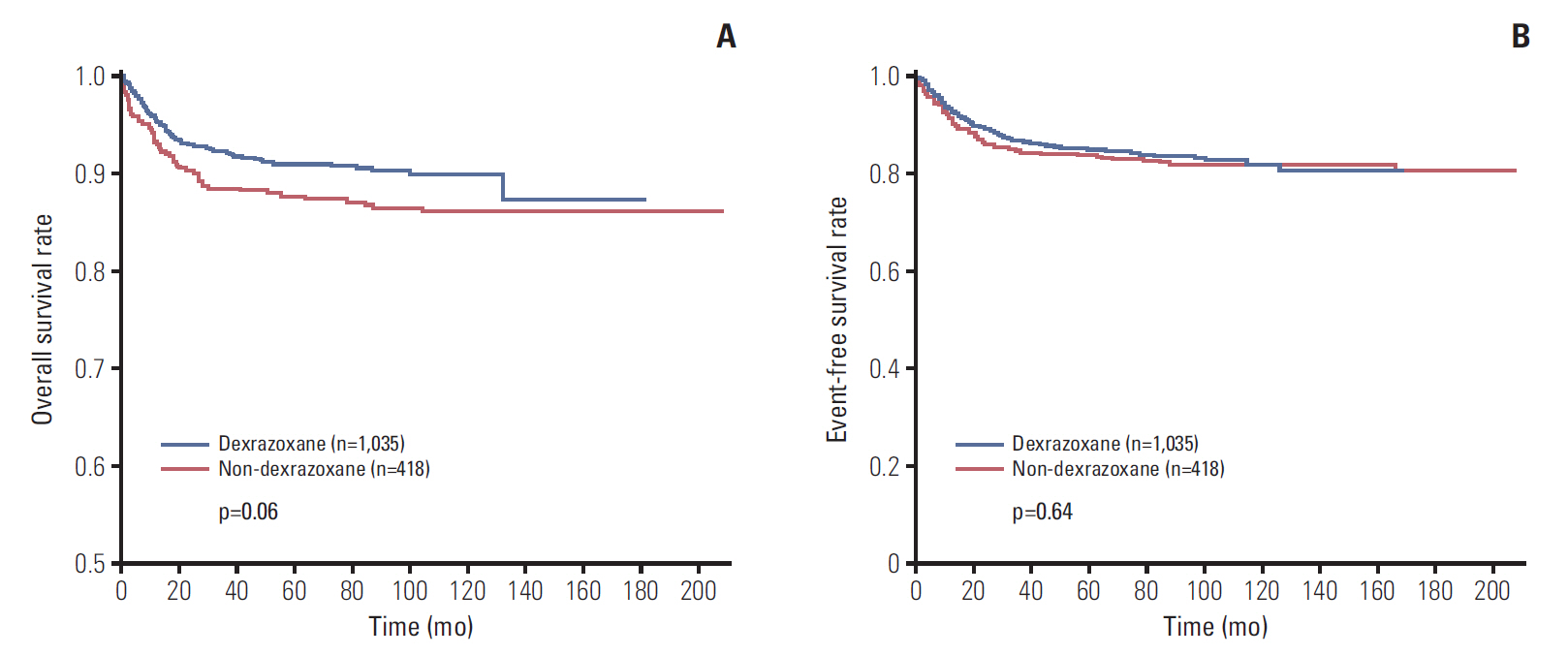
Data of total 1,453 cases were analyzed; dexrazoxane with every anthracyclines group (D group, 1,035 patients) and no dexrazoxane group (non-D group, 418 patients). Incidence of the reported cardiac events was not statistically different between two groups; however, the cardiac event-free survival rate of patients with more than 400 mg/m2 of anthracyclines was significantly higher in D group (91.2% vs. 80.1%, p=0.04). The 6-year cumulative incidence of secondary malignancy was not different between both groups after considering follow-up duration difference (non-D, 0.52%±0.37%; D, 0.60%±0.28%; p=0.55). The most influential risk factor for secondary malignancy was the duration of anthracycline administration according to multivariate analysis.
CONCLUSION
Dexrazoxane had an efficacy in lowering cardiac event-free survival rates in patients with higher cumulative anthracyclines. As a result of multivariate analysis for assessing risk factors of secondary malignancy, the occurrence of secondary malignancy was not related to dexrazoxane administration.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Harake D, Franco VI, Henkel JM, Miller TL, Lipshultz SE. Cardiotoxicity in childhood cancer survivors: strategies for prevention and management. Future Cardiol. 2012; 8:647–70.
Article2. Brewster DH, Clark D, Hopkins L, Bauer J, Wild SH, Edgar AB, et al. Subsequent hospitalisation experience of 5-year survivors of childhood, adolescent, and young adult cancer in Scotland: a population based, retrospective cohort study. Br J Cancer. 2014; 110:1342–50.
Article3. Choi HS, Park ES, Kang HJ, Shin HY, Noh CI, Yun YS, et al. Dexrazoxane for preventing anthracycline cardiotoxicity in children with solid tumors. J Korean Med Sci. 2010; 25:1336–42.
Article4. Barry EV, Vrooman LM, Dahlberg SE, Neuberg DS, Asselin BL, Athale UH, et al. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J Clin Oncol. 2008; 26:1106–11.
Article5. Tebbi CK, London WB, Friedman D, Villaluna D, De Alarcon PA, Constine LS, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol. 2007; 25:493–500.
Article6. Walker DM, Fisher BT, Seif AE, Huang YS, Torp K, Li Y, et al. Dexrazoxane use in pediatric patients with acute lymphoblastic or myeloid leukemia from 1999 and 2009: analysis of a national cohort of patients in the Pediatric Health Information Systems database. Pediatr Blood Cancer. 2013; 60:616–20.
Article7. Vrooman LM, Neuberg DS, Stevenson KE, Asselin BL, Athale UH, Clavell L, et al. The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: a report from the Dana-Farber Cancer Institute ALL Consortium. Eur J Cancer. 2011; 47:1373–9.
Article8. Seif AE, Walker DM, Li Y, Huang YS, Kavcic M, Torp K, et al. Dexrazoxane exposure and risk of secondary acute myeloid leukemia in pediatric oncology patients. Pediatr Blood Cancer. 2015; 62:704–9.
Article9. Blanco JG, Sun CL, Landier W, Chen L, Esparza-Duran D, Leisenring W, et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes: a report from the Children's Oncology Group. J Clin Oncol. 2012; 30:1415–21.10. Florescu M, Cinteza M, Vinereanu D. Chemotherapy-induced cardiotoxicity. Maedica (Buchar). 2013; 8:59–67.11. Kremer LC, van Dalen EC, Offringa M, Ottenkamp J, Voute PA. Anthracycline-induced clinical heart failure in a cohort of 607 children: long-term follow-up study. J Clin Oncol. 2001; 19:191–6.
Article12. Lipshultz SE, Adams MJ. Cardiotoxicity after childhood cancer: beginning with the end in mind. J Clin Oncol. 2010; 28:1276–81.
Article13. Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 2010; 11:950–61.
Article14. Mozdzanowska D, Wozniewski M. Radiotherapy and anthracyclines: cardiovascular toxicity. Contemp Oncol (Pozn). 2015; 19:93–7.15. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003; 97:2869–79.16. Vega-Stromberg T. Chemotherapy-induced secondary malignancies. J Infus Nurs. 2003; 26:353–61.
Article17. Hawkins MM, Wilson LM, Stovall MA, Marsden HB, Potok MH, Kingston JE, et al. Epipodophyllotoxins, alkylating agents, and radiation and risk of secondary leukaemia after childhood cancer. BMJ. 1992; 304:951–8.
Article18. Haddy N, Le Deley MC, Samand A, Diallo I, Guerin S, Guibout C, et al. Role of radiotherapy and chemotherapy in the risk of secondary leukaemia after a solid tumour in childhood. Eur J Cancer. 2006; 42:2757–64.
Article19. Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010; 102:1083–95.
Article20. Choi DK, Helenowski I, Hijiya N. Secondary malignancies in pediatric cancer survivors: perspectives and review of the literature. Int J Cancer. 2014; 135:1764–73.
Article21. Bhuller KS, Zhang Y, Li D, Sehn LH, Goddard K, McBride ML, et al. Late mortality, secondary malignancy and hospitalisation in teenage and young adult survivors of Hodgkin lymphoma: report of the Childhood/Adolescent/Young Adult Cancer Survivors Research Program and the BC Cancer Agency Centre for Lymphoid Cancer. Br J Haematol. 2016; 172:757–68.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Growth hormone treatment and risk of malignancy
- Cardioprotective Effect of Dexrazoxane in Patients with HER2-Positive Breast Cancer Who Receive Anthracycline Based Adjuvant Chemotherapy Followed by Trastuzumab
- Dexrazoxane for Preventing Anthracycline Cardiotoxicity in Children with Solid Tumors
- The Incidence of Occult Malignancy in Contralateral Risk Reducing Mastectomy Among Affected Breast Cancer Gene Mutation Carriers in South Korea
- Secondary Malignancies in Multiple Myeloma in Korean Patients: A Nationwide Population-Based Study