J Korean Med Sci.
2010 Sep;25(9):1336-1342. 10.3346/jkms.2010.25.9.1336.
Dexrazoxane for Preventing Anthracycline Cardiotoxicity in Children with Solid Tumors
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Bundang Hospital, Sungnam, Korea. choi3628@snu.ac.kr
- 2Department of Pediatrics, Gyeongsang National University Hospital, Jinju, Korea.
- 3Department of Pediatrics, Seoul National University Children's Hospital, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1785914
- DOI: http://doi.org/10.3346/jkms.2010.25.9.1336
Abstract
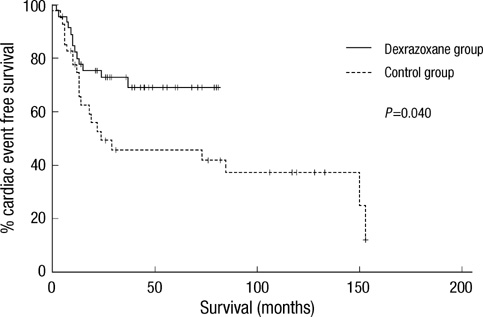
- This study attempted to assess the incidence and outcome of anthracycline cardiotoxicity and the role of dexrazoxane as a cardioprotectant in childhood solid tumors. The dexrazoxane group included 47 patients and the control group of historical cohort included 42. Dexrazoxane was given in the 10:1 ratio to doxorubicin. Fractional shortening and systolic and diastolic left ventricular diameters were used to assess the cardiac function. The median follow-ups were 54 months in the dexrazoxane group and 86 months in the control group. The mean cumulative doses of doxorubicin were 280.8+/-83.4 mg/m2 in the dexrazoxane group and 266.1+/-75.0 mg/m2 in the control group. The dexrazoxane group experienced significantly fewer cardiac events (27.7% vs. 52.4%) and less severe congestive heart failure (6.4% vs. 14.3%) than the control group. Thirteen cardiotoxicities including one cardiac death and 2 congestive heart failures occurred in the dexrazoxane group, and 22 cardiotoxicities including 2 cardiac deaths and 4 congestive heart failures, in the control group. Five year cardiac event free survival rates were 69.2% in the dexrazoxane group and 45.8% in the control group (P=0.04). Dexrazoxane reduces the incidence and severity of early and late anthracycline cardiotoxicity in childhood solid tumors.
Keyword
MeSH Terms
-
Adolescent
Antibiotics, Antineoplastic/*adverse effects
Cardiomyopathies/chemically induced/prevention & control
Cardiovascular Agents/*therapeutic use
Child
Child, Preschool
Cohort Studies
Disease-Free Survival
Doxorubicin/*adverse effects
Echocardiography
Female
Follow-Up Studies
Heart Failure/chemically induced/prevention & control
Humans
Infant
Male
Neoplasms/*drug therapy/mortality
Razoxane/*therapeutic use
Ventricular Function, Left/physiology
Figure
Cited by 1 articles
-
Risk Factor Analysis for Secondary Malignancy in Dexrazoxane-Treated Pediatric Cancer Patients
Hyery Kim, Hyoung Jin Kang, Kyung Duk Park, Kyung-Nam Koh, Ho Joon Im, Jong Jin Seo, Jae Wook Lee, Nack-Gyun Chung, Bin Cho, Hack Ki Kim, Jae Min Lee, Jeong Ok Hah, Jun Ah Lee, Young Ho Lee, Sang Kyu Park, Hee Jo Baek, Hoon Kook, Ji Yoon Kim, Heung Sik Kim, Hwang Min Kim, Hee Won Chueh, Meerim Park, Hoi Soo Yoon, Mee Jeong Lee, Hyoung Soo Choi, Hyo Seop Ahn, Yoshifumi Kawano, Ji Won Park, Seokyung Hahn, Hee Young Shin
Cancer Res Treat. 2019;51(1):357-367. doi: 10.4143/crt.2017.457.
Reference
-
1. van Dalen EC, Caron HN, Kremer LC. Prevention of anthracycline-induced cardiotoxicity in children: the evidence. Eur J Cancer. 2007. 43:1134–1140.
Article2. van Dalen EC, Raphael MF, Caron HN, Kremer LC. Treatment including anthracyclines versus treatment not including anthracyclines for childhood cancer. Cochrane Database Syst Rev. 2009. (1):CD006647.
Article3. Krischer JP, Epstein S, Cuthbertson DD, Goorin AM, Epstein ML, Lipshultz SE. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol. 1997. 15:1544–1552.
Article4. Kwon HJ, Song YH, Kang SJ, Kang HJ, Choi HS, Bae EJ, Shin HY, Noh CI, Yun YS, Ahn HS. Follow-up study of children with anthracycline cardiotoxicity. J Korean Pediatr Soc. 2003. 46:242–249.5. Wexler LH, Andrich MP, Venzon D, Berg SL, Weaver-McClure L, Chen CC, Dilsizian V, Avila N, Jarosinski P, Balis FM, Poplack DG, Horowitz ME. Randomized trial of the cardioprotective agent ICRF-187 in pediatric sarcoma patients treated with doxorubicin. J Clin Oncol. 1996. 14:362–372.
Article6. Sorensen K, Levitt G, Bull C, Chessells J, Sullivan I. Anthracycline dose in childhood acute lymphoblastic leukemia: issues of early survival versus late cardiotoxicity. J Clin Oncol. 1997. 15:61–68.
Article7. Sorensen K, Levitt GA, Bull C, Dorup I, Sullivan ID. Late anthracycline cardiotoxicity after childhood cancer: a prospective longitudinal study. Cancer. 2003. 97:1991–1998.8. Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, Colan SD. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005. 23:2629–2636.
Article9. Elbl L, Hrstkova H, Tomaskova I, Blazek B, Michalek J. Long-term serial echocardiographic examination of late anthracycline cardiotoxicity and its prevention by dexrazoxane in paediatric patients. Eur J Pediatr. 2005. 164:678–684.
Article10. van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2008. (2):CD003917.
Article11. Cvetkovic RS, Scott LJ. Dexrazoxane: a review of its use for cardioprotection during anthracycline chemotherapy. Drugs. 2005. 65:1005–1024.12. Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, Cohen GI, Emami B, Gradishar WJ, Mitchell RB, Thigpen JT, Trotti A 3rd, von Hoff D, Schuchter LM. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009. 27:127–145.
Article13. Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, Colan SD, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A, Samson Y, Schorin MA, Gelber RD, Sallan SE. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004. 351:145–153.
Article14. Schuchter LM, Hensley ML, Meropol NJ, Winer EP. 2002 update of recommendations for the use of chemotherapy and radiotherapy protectants: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2002. 20:2895–2903.
Article15. Henry WL. Evaluation of ventricular function using two dimensional echocardiography. Am J Cardiol. 1982. 49:1319–1323.
Article16. Henry WL, Gardin JM, Ware JH. Echocardiographic measurements in normal subjects from infancy to old age. Circulation. 1980. 62:1054–1061.
Article17. Mertens AC, Yasui Y, Neglia JP, Potter JD, Nesbit ME Jr, Ruccione K, Smithson WA, Robison LL. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001. 19:3163–3172.
Article18. Kremer LC, van Dalen EC, Offringa M, Voute PA. Frequency and risk factors of anthracycline-induced clinical heart failure in children: a systematic review. Ann Oncol. 2002. 13:503–512.
Article19. Kremer LC, van der Pal HJ, Offringa M, van Dalen EC, Voute PA. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann Oncol. 2002. 13:819–829.
Article20. Marty M, Espie M, Llombart A, Monnier A, Rapoport BL, Stahalova V. Dexrazoxane Study Group. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Ann Oncol. 2006. 17:614–622.
Article21. Elbl L, Hrstkova H, Tomaskova I, Michalek J. Late anthracycline cardiotoxicity protection by dexrazoxane (ICRF-187) in pediatric patients: echocardiographic follow-up. Support Care Cancer. 2006. 14:128–136.
Article22. Kovacs GT, Erlaky H, Toth K, Horváth E, Szabolcs J, Csoka M, Jokuti L, Erdelyi D, Muller J. Subacute cardiotoxicity caused by anthracycline therapy in children: can dexrazoxane prevent this effect? Eur J Pediatr. 2007. 166:1187–1188.
Article23. Nysom K, Holm K, Lipsitz SR, Mone SM, Colan SD, Orav EJ, Sallan SE, Olsen JH, Hertz H, Jacobsen JR, Lipshultz SE. Relationship between cumulative anthracycline dose and late cardiotoxicity in childhood acute lymphoblastic leukemia. J Clin Oncol. 1998. 16:545–550.
Article24. van Dalen EC, van der Pal HJ, Kok WE, Caron HN, Kremer LC. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer. 2006. 42:3191–3198.
Article25. Lipshultz SE, Rifai N, Sallan SE, Lipsitz SR, Dalton V, Sacks DB, Ottlinger ME. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation. 1997. 96:2641–2648.
Article26. Ruggiero A, Ridola V, Puma N, Molinari F, Coccia P, De Rosa G, Riccardi R. Anthracycline cardiotoxicity in childhood. Pediatr Hematol Oncol. 2008. 25:261–281.
Article27. Meinardi MT, van der Graaf WT, van Veldhuisen DJ, Gietema JA, de Vries EG, Sleijfer DT. Detection of anthracycline-induced cardiotoxicity. Cancer Treat Rev. 1999. 25:237–247.
Article28. Tebbi CK, London WB, Friedman D, Villaluna D, De Alarcon PA, Constine LS, Mendenhall NP, Sposto R, Chauvenet A, Schwartz CL. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol. 2007. 25:493–500.
Article29. Barry EV, Vrooman LM, Dahlberg SE, Neuberg DS, Asselin BL, Athale UH, Clavell LA, Larsen EC, Moghrabi A, Samson Y, Schorin MA, Cohen HJ, Lipshultz SE, Sallan SE, Silverman LB. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J Clin Oncol. 2008. 26:1106–1111.
Article30. Bryant J, Picot J, Baxter L, Levitt G, Sullivan I, Clegg A. Clinical and cost-effectiveness of cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review. Br J Cancer. 2007. 96:226–230.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathophysiology and preventive strategies of anthracycline-induced cardiotoxicity
- Follow-up Study of Children with Anthracycline Cardiotoxicity
- Clinical Correlation between Brain Natriutetic Peptide and Anthracyclin-induced Cardiac Toxicity
- Cardioprotective Effect of Dexrazoxane in Patients with HER2-Positive Breast Cancer Who Receive Anthracycline Based Adjuvant Chemotherapy Followed by Trastuzumab
- Risk Factor Analysis for Secondary Malignancy in Dexrazoxane-Treated Pediatric Cancer Patients