Chonnam Med J.
2019 Jan;55(1):31-39. 10.4068/cmj.2019.55.1.31.
IL-12 Enhances Immune Response by Modulation of Myeloid Derived Suppressor Cells in Tumor Microenvironment
- Affiliations
-
- 1Department of Hematology-Oncology, Chonnam National University Hwasun Hospital, Hwasun, Korea. shcho@jnu.ac.kr
- KMID: 2432253
- DOI: http://doi.org/10.4068/cmj.2019.55.1.31
Abstract
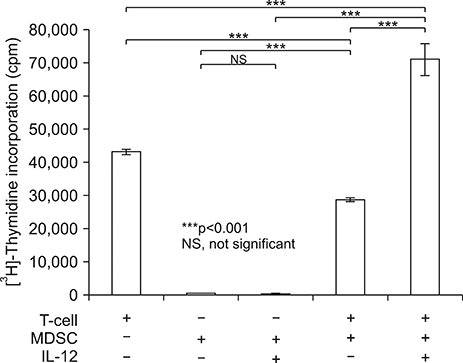
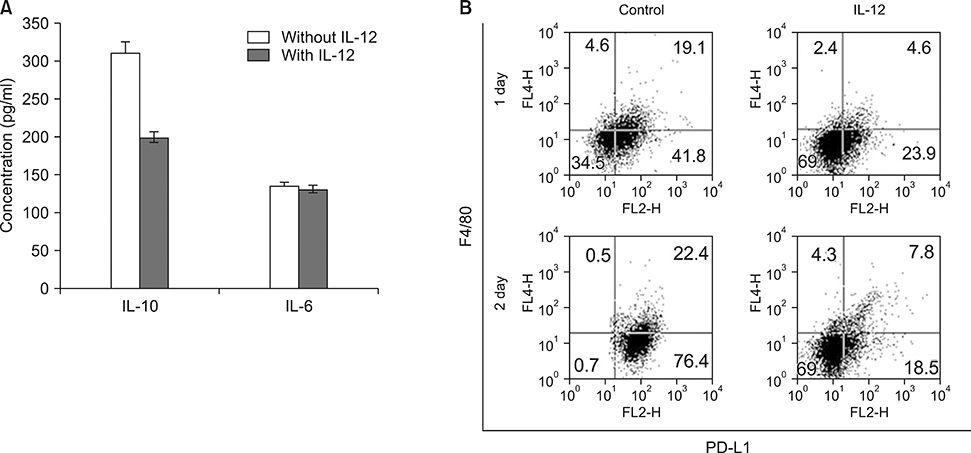
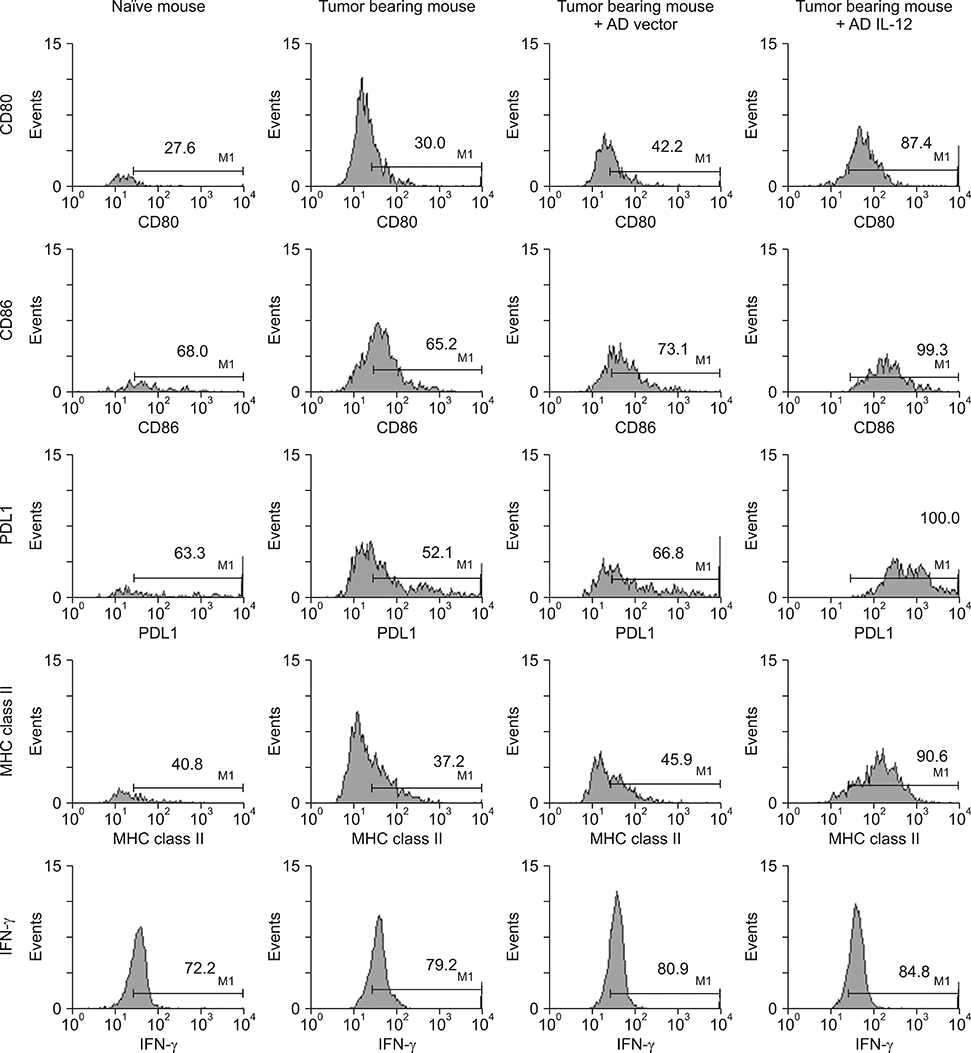
- Myeloid derived suppressor cells (MDSCs) are a heterogenous population of immature cells that play a critical role in tumor associated immune suppression. In tumor conditions, the population of MDSCs increases. The main feature of these cells is their ability to suppress the T cell response in antigen specific or nonspecific manners depending on the condition of T cell activation. IL-12 can modulate MDSC in preliminary reports, so we investigated how IL-12 can affect MDSC in a tumor microenvironment. After implanting tumor based cells on syngeneic host, 4T-1/BALB/c or EL4/C57BL6 mice, MDSCs (Gr1+CD11b+) were isolated from splenocytes. Isolated MDSCs were treated with GM-CSF with or without IL-12 and analyzed based on their phenotypes and functions. Treatment of MDSC with IL-12 increased co-stimulatory molecules of CD80, CD86, OX-40L, enhancing the DC phenotype (CD11c) and maturation markers such as p-NF-κB and p-GSK3β. In addition to a change of surface markers, T-cell suppressive function of MDSC after IL-12 treatment was significantly improved compared with the control MDSC. In addition, PD-L1+F4/80+ macrophages, which show aninhibitory effect in phagocytosis, were decreased after IL-12 treatment. The changes of cell surface expression of CD80, CD86, MHC class II were also shown in vivo. Our results showed that the IL-12 can modulate MDSC into APC and recover the macrophage function. These results suggested that IL-12 plays a role in improving the tumor immune microenvironment through MDSC modulation.
MeSH Terms
Figure
Reference
-
1. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009; 9:162–174.
Article2. Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008; 181:5791–5802.
Article3. Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010; 185:2273–2284.
Article4. Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016; 37:208–220.
Article5. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012; 12:253–268.
Article6. Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005; 5:641–654.
Article7. Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007; 13:828–835.
Article8. Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012; 13:722–728.
Article9. Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006; 177:7515–7519.
Article10. Mazzolini G, Alfaro C, Sangro B, Feijoó E, Ruiz J, Benito A, et al. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J Clin Oncol. 2005; 23:999–1010.
Article11. Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006; 42:717–727.
Article12. Ma X, Yan W, Zheng H, Du Q, Zhang L, Ban Y, et al. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Res. 2015; 4:pii: F1000 Faculty Rev-1465.
Article13. Steding CE, Wu ST, Zhang Y, Jeng MH, Elzey BD, Kao C. The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis. Immunology. 2011; 133:221–238.
Article14. Alessandrini A, De Haseth S, Fray M, Miyajima M, Colvin RB, Williams WW, et al. Dendritic cell maturation occurs through the inhibition of GSK-3β. Cell Immunol. 2011; 270:114–125.
Article15. Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017; 545:495–499.
Article16. Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009; 9:900–909.
Article17. Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010; 70:3052–3061.
Article18. Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009; 15:2148–2157.
Article19. Bauer R, Udonta F, Wroblewski M, Ben-Batalla I, Santos IM, Taverna F, et al. Blockade of myeloid-derived suppressor cell expansion with all-trans retinoic acid increases the efficacy of antiangiogenic Therapy. Cancer Res. 2018; 78:3220–3232.
Article20. Smyth MJ, Taniguchi M, Street SE. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000; 165:2665–2670.
Article21. Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011; 121:4746–4757.
Article22. Bottero V, Withoff S, Verma IM. NF-kappaB and the regulation of hematopoiesis. Cell Death Differ. 2006; 13:785–797.23. Zhang TY, Daynes RA. Glucocorticoid conditioning of myeloid progenitors enhances TLR4 signaling via negative regulation of the phosphatidylinositol 3-kinase-Akt pathway. J Immunol. 2007; 178:2517–2526.
Article24. Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI, Park YM, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274). FEBS Lett. 2006; 580:755–762.
Article25. Nastala CL, Edington HD, McKinney TG, Tahara H, Nalensnik MA, Brunda MJ, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994; 153:1697–1706.26. Tang H, Liang Y, Anders RA, Taube JM, Qiu X, Mulgaonkar A, et al. PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J Clin Invest. 2018; 128:580–588.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interactions between Immune Cells and Tumor Cells
- Modulation of Immunosuppression by Oligonucleotide-Based Molecules and Small Molecules Targeting Myeloid-Derived Suppressor Cells
- Exploring the Potential of Glycolytic Modulation in Myeloid-Derived Suppressor Cells for Immunotherapy and Disease Management
- Deciphering and Reversing Immunosuppressive Cells in the Treatment of Hepatocellular Carcinoma
- Analysis of the Expression and Regulation of PD-1 Protein on the Surface of Myeloid-Derived Suppressor Cells (MDSCs)