Yonsei Med J.
2019 Feb;60(2):132-139. 10.3349/ymj.2019.60.2.132.
Single Patient Classifier Assay, Microsatellite Instability, and Epstein-Barr Virus Status Predict Clinical Outcomes in Stage II/III Gastric Cancer: Results from CLASSIC Trial
- Affiliations
-
- 1Department of Surgery, Yonsei University Health System, Seoul, Korea. JHCHEONG@yuhs.ac
- 2Yonsei Biomedical Research Institute, Yonsei University Health System, Seoul, Korea.
- 3MediBio-Informatics Research Center, Novomics Co., Ltd., Seoul, Korea.
- 4Department of Radiology, Yonsei University Health System, Seoul, Korea.
- 5Department of Biochemistry & Molecular Biology, Yonsei University Health System, Seoul, Korea.
- 6YUHS-KRIBB Medical Convergence Research Institute, Seoul, Korea.
- 7Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2431629
- DOI: http://doi.org/10.3349/ymj.2019.60.2.132
Abstract
- PURPOSE
Clinical implications of single patient classifier (SPC) and microsatellite instability (MSI) in stage II/III gastric cancer have been reported. We investigated SPC and the status of MSI and Epstein-Barr virus (EBV) as combinatory biomarkers to predict the prognosis and responsiveness of adjuvant chemotherapy for stage II/III gastric cancer.
MATERIALS AND METHODS
Tumor specimens and clinical information were collected from patients enrolled in CLASSIC trial, a randomized controlled study of capecitabine plus oxaliplatin-based adjuvant chemotherapy. The results of nine-gene based SPC assay were classified as prognostication (SPC-prognosis) and prediction of chemotherapy benefit (SPC-prediction). Five quasimonomorphic mononucleotide markers were used to assess tumor MSI status. EBV-encoded small RNA in situ hybridization was performed to define EBV status.
RESULTS
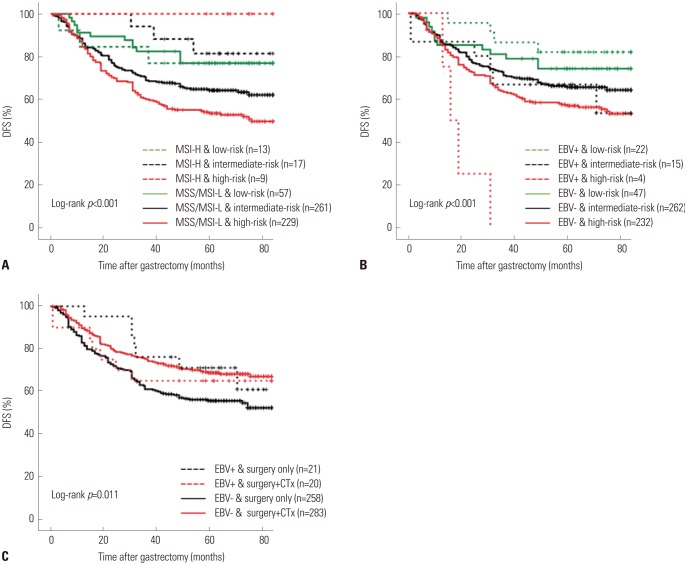
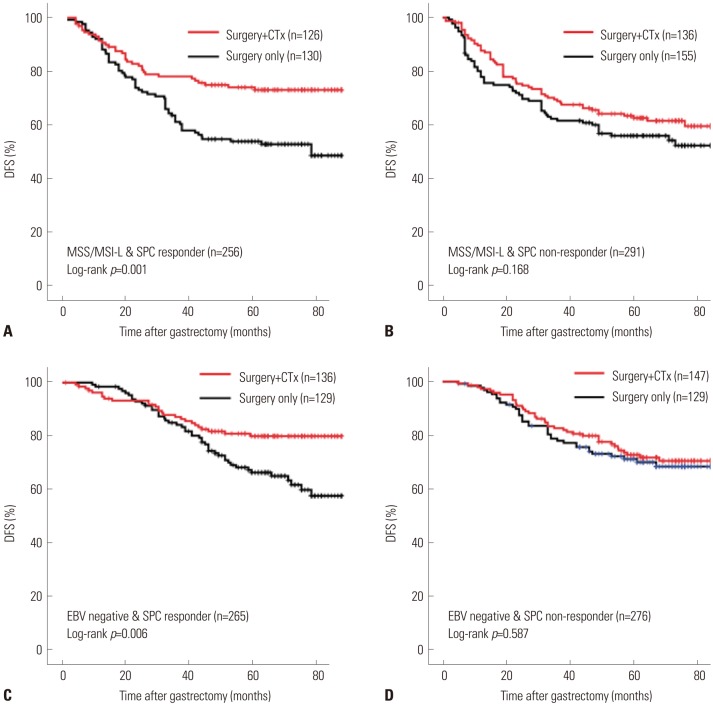
There were positive associations among SPC, MSI, and EBV statuses among 586 patients. In multivariate analysis of disease-free survival, SPC-prognosis [hazard ratio (HR): 1.879 (1.101-3.205), 2.399 (1.415-4.067), p=0.003] and MSI status (HR: 0.363, 95% confidence interval: 0.161-0.820, p=0.015) were independent prognostic factors along with age, Lauren classification, TNM stage, and chemotherapy. Patient survival of SPC-prognosis was well stratified regardless of EBV status and in microsatellite stable (MSS) group, but not in MSI-high group. Significant survival benefit from adjuvant chemotherapy was observed by SPC-Prediction in MSS and EBV-negative gastric cancer.
CONCLUSION
SPC, MSI, and EBV statuses could be used in combination to predict the prognosis and responsiveness of adjuvant chemotherapy for stage II/III gastric cancer.
MeSH Terms
Figure
Cited by 2 articles
-
Ten Thousand Consecutive Gastrectomies for Gastric Cancer: Perspectives of a Master Surgeon
Yoon Young Choi, Minah Cho, In Gyu Kwon, Taeil Son, Hyoung-Il Kim, Seung Ho Choi, Jae-Ho Cheong, Woo Jin Hyung
Yonsei Med J. 2019;60(3):235-242. doi: 10.3349/ymj.2019.60.3.235.A Multi-cohort Study of the Prognostic Significance of Microsatellite Instability or Mismatch Repair Status after Recurrence of Resectable Gastric Cancer
Ji Yeong An, Yoon Young Choi, Jeeyun Lee, Woo Jin Hyung, Kyoung-Mee Kim, Sung Hoon Noh, Min-Gew Choi, Jae-Ho Cheong
Cancer Res Treat. 2020;52(4):1153-1161. doi: 10.4143/crt.2020.173.
Reference
-
1. Choi YY, Cheong JH. Beyond precision surgery: molecularly motivated precision care for gastric cancer. Eur J Surg Oncol. 2017; 43:856–864. PMID: 28330821.
Article2. Choi YY, Noh SH, Cheong JH. Evolution of gastric cancer treatment: from the golden age of surgery to an era of precision medicine. Yonsei Med J. 2015; 56:1177–1185. PMID: 26256958.
Article3. Choi YY, Noh SH, Cheong JH. Molecular dimensions of gastric cancer: translational and clinical perspectives. J Pathol Transl Med. 2016; 50:1–9. PMID: 26498010.
Article4. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–697. PMID: 20728210.
Article5. Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003; 349:247–257. PMID: 12867608.
Article6. Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004; 351:2817–2826. PMID: 15591335.
Article7. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014; 513:202–209. PMID: 25079317.8. Tan IB, Ivanova T, Lim KH, Ong CW, Deng N, Lee J, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011; 141:476–485. PMID: 21684283.
Article9. Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015; 21:449–456. PMID: 25894828.
Article10. Choi YY, Bae JM, An JY, Kwon IG, Cho I, Shin HB, et al. Is microsatellite instability a prognostic marker in gastric cancer? A systematic review with meta-analysis. J Surg Oncol. 2014; 110:129–135. PMID: 24737677.
Article11. Kim SY, Choi YY, An JY, Shin HB, Jo A, Choi H, et al. The benefit of microsatellite instability is attenuated by chemotherapy in stage II and stage III gastric cancer: results from a large cohort with subgroup analyses. Int J Cancer. 2015; 137:819–825. PMID: 25614197.
Article12. Smyth EC, Wotherspoon A, Peckitt C, Gonzalez D, Hulkki-Wilson S, Eltahir Z, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol. 2017; 3:1197–1203. PMID: 28241187.13. Choi YY, Kim H, Shin SJ, Kim HY, Lee J, Yang HK, et al. Microsatellite instability and programmed cell death-ligand 1 expression in stage II/III gastric cancer: post hoc analysis of the CLASSIC randomized controlled study. Ann Surg. 2018; 5. 01. [Epub]. DOI: 10.1097/SLA.0000000000002803.14. Choi YY, Cheong JH. To treat, or not to treat, that is the question: biomarker-guided adjuvant chemotherapy for stage II and III gastric cancer. Ann Surg. 2018; 11. 22. [Epub]. DOI: 10.1097/SLA.0000000000003102.15. Cheong JH, Yang HK, Kim H, Kim WH, Kim YW, Kook MC, et al. Predictive test for chemotherapy response in resectable gastric cancer: a multi-cohort, retrospective analysis. Lancet Oncol. 2018; 19:629–638. PMID: 29567071.
Article16. Gambardella V, Cervantes A. Precision medicine in the adjuvant treatment of gastric cancer. Lancet Oncol. 2018; 19:583–584. PMID: 29567072.
Article17. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012; 379:315–321. PMID: 22226517.
Article18. Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014; 15:1389–1396. PMID: 25439693.
Article19. Patil DT, Bronner MP, Portier BP, Fraser CR, Plesec TP, Liu X. A five-marker panel in a multiplex PCR accurately detects microsatellite instability-high colorectal tumors without control DNA. Diagn Mol Pathol. 2012; 21:127–133. PMID: 22847155.
Article20. Goel A, Nagasaka T, Hamelin R, Boland CR. An optimized pentaplex PCR for detecting DNA mismatch repair-deficient colorectal cancers. PLoS One. 2010; 5:e9393. PMID: 20195377.
Article21. Kim HS, Shin SJ, Beom SH, Jung M, Choi YY, Son T, et al. Comprehensive expression profiles of gastric cancer molecular subtypes by immunohistochemistry: implications for individualized therapy. Oncotarget. 2016; 7:44608–44620. PMID: 27331626.
Article22. Park JH, Kim EK, Kim YH, Kim JH, Bae YS, Lee YC, et al. Epstein-Barr virus positivity, not mismatch repair-deficiency, is a favorable risk factor for lymph node metastasis in submucosa-invasive early gastric cancer. Gastric Cancer. 2016; 19:1041–1051. PMID: 26573601.
Article23. Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018; 24:1449–1458. PMID: 30013197.
Article24. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006; 355:11–20. PMID: 16822992.
Article25. Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim KM, et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: final report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, including survival and subset analyses. J Clin Oncol. 2015; 33:3130–3136. PMID: 25559811.
Article26. Al-Batran SE, Pauligk C, Homann N, Schmalenberg H, Kopp HG, Haag GM, et al. LBA-008: Docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) as perioperative treatment of resectable gastric or gastro-esophageal junction adenocarcinoma: the multicenter, randomized phase 3 FLOT4 trial (German Gastric Group at AIO). Ann Oncol. 2017; 28(suppl_3):mdx302.007.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is a Microsatellite Instability Still Useful for Tailored Treatment in Stage II and III Colon Cancer?
- Genomic assays for Epstein-Barr virus-positive gastric adenocarcinoma
- The Predictive Value of Epstein-Barr Virus-Positivity in Patients Undergoing Gastrectomy Followed by Adjuvant Chemotherapy
- Epstein-Barr Virus in Human Malignancy: A Special Reference to Epstein-Barr Virus associated Gastric Carcinoma
- A Case of Epstein-Barr Virus-Positive Diffuse Large B-Cell Lymphoma Occurring in Thyroid Gland