Chonnam Med J.
2018 Sep;54(3):173-177. 10.4068/cmj.2018.54.3.173.
The Predictive Value of Epstein-Barr Virus-Positivity in Patients Undergoing Gastrectomy Followed by Adjuvant Chemotherapy
- Affiliations
-
- 1Department of Oncology/Hematology, Kyungpook National University Chilgok Hospital, School of Medicine, Kyungpook National University, Kyungpook National University Cancer Research Institute, Daegu, Korea. bwkang@knu.ac.kr, jkk21c@knu.ac.kr
- KMID: 2420885
- DOI: http://doi.org/10.4068/cmj.2018.54.3.173
Abstract
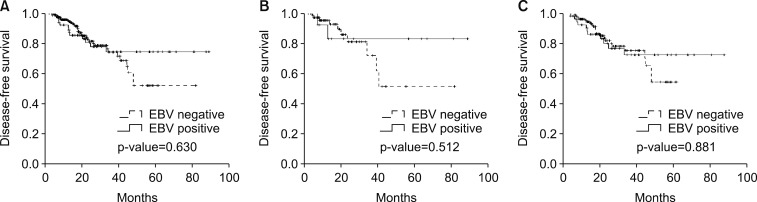
- The present study evaluated the survival impact of standard adjuvant chemotherapy and prognostic differences between Epstein-Barr virus (EBV)-associated gastric cancer (EBVaGC) and EBV-negative gastric cancer (EBVnGC). A total of 276 patients were enrolled according to the following criteria: 1) pathologically diagnosed with primary gastric adenocarcinoma, 2) test results from EBV-encoded RNA in situ hybridization, 3) stage II/III according to the 7th edition of UICC/AJCC staging system for gastric cancer, and 4) postoperative adjuvant chemotherapy. Fifty-nine (21.4%) and 217 (78.6%) patients exhibited EBVaGC and EBVnGC, respectively, while 129 (46.7%) patients were classified as stage II and 147 (53.3%) as stage III. As for adjuvant chemotherapy, 87 (31.5%) patients received capecitabine and oxaliplatin, while 189 (68.5%) received S-1 monotherapy. With a median follow-up duration of 21.3 (6.4-89.0) months, the estimated 3-year disease-free survival (DFS) and overall survival (OS) rates were 74.8% and 83.0%, respectively. In univariate analysis and multivariate analysis using a Cox proportional hazard model including age, gender, stage, Lauren classification, and the type of chemotherapy, EBV-positivity was not significantly associated with DFS (p-value= 0.630) regardless of the type of chemotherapy. Therefore, no association was found between EBV positivity and the survival outcomes in patients with curatively resected gastric cancer who received standard adjuvant chemotherapy.
MeSH Terms
Figure
Reference
-
1. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014; 513:202–209. PMID: 25079317.2. Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009; 137:824–833. PMID: 19445939.3. zur Hausen A, van Grieken NC, Meijer GA, Hermsen MA, Bloemena E, Meuwissen SG, et al. Distinct chromosomal aberrations in Epstein-Barr virus-carrying gastric carcinomas tested by comparative genomic hybridization. Gastroenterology. 2001; 121:612–618. PMID: 11522745.4. Wu MS, Shun CT, Wu CC, Hsu TY, Lin MT, Chang MC, et al. Epstein-Barr virus-associated gastric carcinomas: relation to H. pylori infection and genetic alterations. Gastroenterology. 2000; 118:1031–1038. PMID: 10833477.5. van Beek J, zur Hausen A, Klein Kranenbarg E, van de Velde CJ, Middeldorp JM, van den Brule AJ, et al. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004; 22:664–670. PMID: 14966089.6. Shinozaki-Ushiku A, Kunita A, Fukayama M. Update on Epstein-Barr virus and gastric cancer (review). Int J Oncol. 2015; 46:1421–1434. PMID: 25633561.7. Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014; 15:1389–1396. PMID: 25439693.8. GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group. Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010; 303:1729–1737. PMID: 20442389.9. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007; 357:1810–1820. PMID: 17978289.10. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011; 29:4387–4393. PMID: 22010012.11. Sohn BH, Hwang JE, Jang HJ, Lee HS, Oh SC, Shim JJ, et al. Clinical significance of four molecular subtypes of gastric cancer identified by the cancer genome atlas project. Clin Cancer Res. 2017; [Epub ahead of print]. DOI: 10.1158/1078-0432.CCR-16-2211.12. Aoyama T, Yoshikawa T, Watanabe T, Hayashi T, Ogata T, Cho H, et al. Survival and prognosticators of gastric cancer that recurs after adjuvant chemotherapy with S-1. Gastric Cancer. 2011; 14:150–154. PMID: 21327443.13. Wang K, Kan J, Yuen ST, Shi ST, Chu KM, Law S, et al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011; 43:1219–1223. PMID: 22037554.14. Zang ZJ, Cutcutache I, Poon SL, Zhang SL, McPherson JR, Tao J, et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet. 2012; 44:570–574. PMID: 22484628.15. Zouridis H, Deng N, Ivanova T, Zhu Y, Wong B, Huang D, et al. Methylation subtypes and large-scale epigenetic alterations in gastric cancer. Sci Transl Med. 2012; 4:156ra140.16. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474. PMID: 20180029.17. Sukawa Y, Yamamoto H, Nosho K, Kunimoto H, Suzuki H, Adachi Y, et al. Alterations in the human epidermal growth factor receptor 2-phosphatidylinositol 3-kinase-v-Akt pathway in gastric cancer. World J Gastroenterol. 2012; 18:6577–6586. PMID: 23236232.18. Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015; 21:449–456. PMID: 25894828.19. Choi YY, Kim H, Shin SJ, Kim HY, Lee J, Yang HK, et al. Microsatellite instability and programmed cell death-ligand 1 expression in stage II/III gastric cancer: post hoc analysis of the CLASSIC randomized controlled study. Ann Surg. 2018; [Epub ahead of print]. DOI: 10.1097/SLA.0000000000002803.20. Seo JS, Kim TG, Hong YS, Chen JY, Lee SK. Contribution of Epstein-Barr virus infection to chemoresistance of gastric carcinoma cells to 5-fluorouracil. Arch Pharm Res. 2011; 34:635–643. PMID: 21544729.21. Shin HJ, Kim DN, Lee SK. Association between Epstein-Barr virus infection and chemoresistance to docetaxel in gastric carcinoma. Mol Cells. 2011; 32:173–179. PMID: 21626300.22. Song HJ, Srivastava A, Lee J, Kim YS, Kim KM, Ki Kang W, et al. Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology. 2010; 139:84–92. PMID: 20398662.23. Huang SC, Ng KF, Chen KH, Hsu JT, Liu KH, Yeh TS, et al. Prognostic factors in Epstein-Barr virus-associated stage I-III gastric carcinoma: implications for a unique type of carcinogenesis. Oncol Rep. 2014; 32:530–538. PMID: 24899228.24. Naseem M, Barzi A, Brezden-Masley C, Puccini A, Berger MD, Tokunaga R, et al. Outlooks on Epstein-Barr virus associated gastric cancer. Cancer Treat Rev. 2018; 66:15–22. PMID: 29631196.25. Huang SCM, Tsao SW, Tsang CM. Interplay of viral infection, host cell factors and tumor microenvironment in the pathogenesis of nasopharyngeal carcinoma. Cancers (Basel). 2018; 10:E106. PMID: 29617291.26. Fu DG. Epigenetic alterations in gastric cancer (review). Mol Med Rep. 2015; 12:3223–3230. PMID: 25997695.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Epstein-Barr Virus-Positive Diffuse Large B-Cell Lymphoma Occurring in Thyroid Gland
- A Case of Epstein-Barr Virus-Associated Hemophagocytic Syndrome Demonstrated by In Situ Hybridization
- Studies on the Epstein-Barr virus transformed human B-lymphocytes1. flowcytometry analysis of Epstein-Barr virus transformed human B-lymphocytes
- Studies on the Epstein-Barr virus transformed human B-lymphocytes2. production of LT-like factor by Epstein-Barr virus transformed human B-lymphocytes
- Several Cases of Epstein-Barr Virus Associated Hepatitis