Korean J Physiol Pharmacol.
2018 Nov;22(6):713-719. 10.4196/kjpp.2018.22.6.713.
Potentiation of endothelium-dependent vasorelaxation of mesenteric arteries from spontaneously hypertensive rats by gemigliptin, a dipeptidyl peptidase-4 inhibitor class of anti-diabetic drug
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul 03080, Korea. sjoonkim@snu.ac.kr
- 2Hypoxic/Ischemic Disease Institute, Seoul National University College of Medicine, Seoul 03080, Korea.
- 3Department of Regulatory Toxicology, Life Science R&D, LG Chem Ltd., LG Science Park, Seoul 07796, Korea. baekeunbok@hanmail.net
- KMID: 2430094
- DOI: http://doi.org/10.4196/kjpp.2018.22.6.713
Abstract
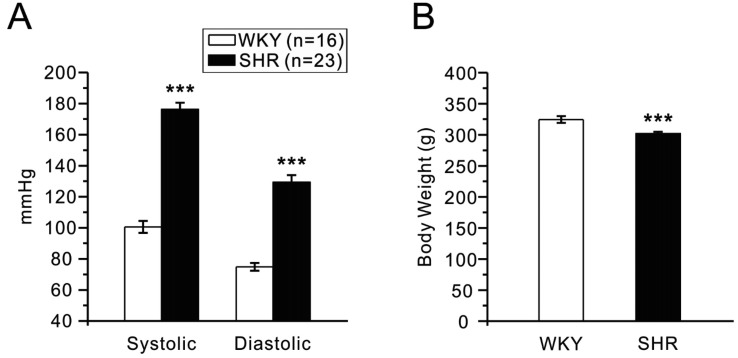
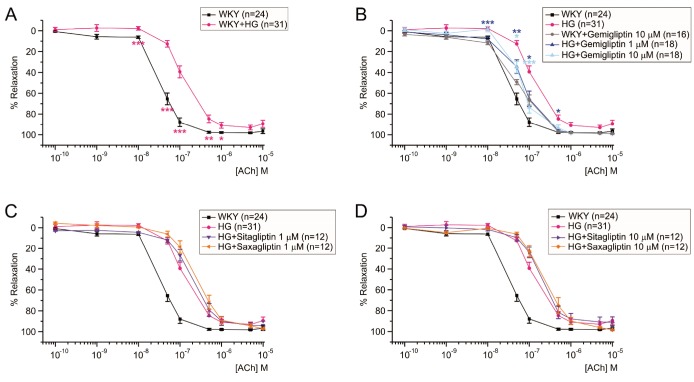
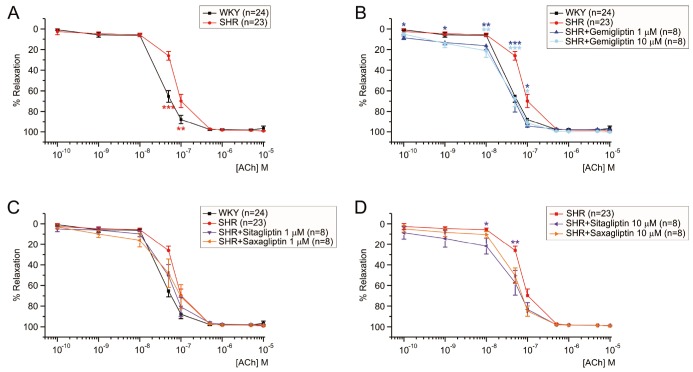
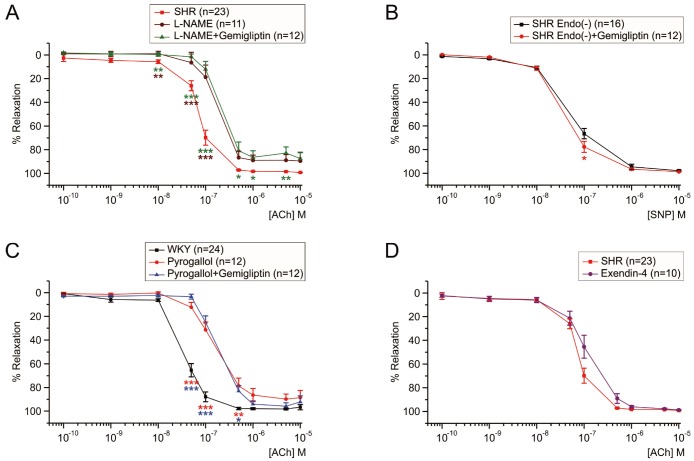
- Dipeptidyl peptidase4 (DPP4) inhibitors such as gemigliptin are anti-diabetic drugs elevating plasma concentration of incretins such as GLP-1. In addition to the DPP4 inhibition, gemigliptin might directly improve the functions of vessels under pathological conditions. To test this hypothesis, we investigated whether the acetylcholine-induced endothelium dependent relaxation (ACh-EDR) of mesenteric arteries (MA) are altered by gemigliptin pretreatment in Spontaneous Hypertensive Rats (SHR) and in Wistar-Kyoto rats (WKY) under hyperglycemia-like conditions (HG; 2 hr incubation with 50 mM glucose). ACh-EDR of WKY was reduced by the HG condition, which was significantly recovered by 1 µM gemigliptin while not by saxagliptin and sitagliptin up to 10 µM. The ACh-EDR of SHR MA was also improved by 1 µM gemigliptin while similar recovery was observed with higher concentration (10 µM) of saxagliptin and sitagliptin. The facilitation of ACh-EDR by gemigliptin in SHR was not observed under pretreatment with NOS inhibitor, L-NAME. In the endotheliumdenuded MA of SHR, sodium nitroprusside induced dose-dependent relaxation was not affected by gemigliptin. The ACh-EDR in WKY was decreased by treatment with 30 µM pyrogallol, a superoxide generator, which was not prevented by gemigliptin. Exendin-4, a GLP-1 analogue, could not enhance the ACh-EDR in SHR MA. The present results of ex vivo study suggest that gemigliptin enhances the NOS-mediated EDR of the HG-treated MA as well as the MA from SHR via GLP-1 receptor independent mechanism.
Keyword
MeSH Terms
-
Animals
Endothelium
Glucagon-Like Peptide 1
Glucagon-Like Peptide-1 Receptor
Hyperglycemia
Hypertension
Incretins
Mesenteric Arteries*
NG-Nitroarginine Methyl Ester
Nitroprusside
Plasma
Pyrogallol
Rats
Rats, Inbred SHR*
Relaxation
Sitagliptin Phosphate
Superoxides
Vasodilation*
Glucagon-Like Peptide 1
Glucagon-Like Peptide-1 Receptor
Incretins
NG-Nitroarginine Methyl Ester
Nitroprusside
Pyrogallol
Sitagliptin Phosphate
Superoxides
Figure
Reference
-
1. Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, Wolfson SK. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993; 88:837–845. PMID: 8353913.
Article2. Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014; 5:444–470. PMID: 25126392.
Article3. Ahrén B. Dipeptidyl peptidase-4 inhibitors: clinical data and clinical implications. Diabetes Care. 2007; 30:1344–1350. PMID: 17337494.4. Papagianni M, Tziomalos K. Cardiovascular effects of dipeptidyl peptidase-4 inhibitors. Hippokratia. 2015; 19:195–199. PMID: 27418775.5. Fadini GP, Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vascul Pharmacol. 2011; 55:10–16. PMID: 21664294.
Article6. Sauvé M, Ban K, Momen MA, Zhou YQ, Henkelman RM, Husain M, Drucker DJ. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes. 2010; 59:1063–1073. PMID: 20097729.
Article7. Esposito G, Cappetta D, Russo R, Rivellino A, Ciuffreda LP, Roviezzo F, Piegari E, Berrino L, Rossi F, De Angelis A, Urbanek K. Sitagliptin reduces inflammation, fibrosis and preserves diastolic function in a rat model of heart failure with preserved ejection fraction. Br J Pharmacol. 2017; 174:4070–4086. PMID: 27922176.
Article8. Koibuchi N, Hasegawa Y, Katayama T, Toyama K, Uekawa K, Sueta D, Kusaka H, Ma M, Nakagawa T, Lin B, Kim-Mitsuyama S. DPP-4 inhibitor linagliptin ameliorates cardiovascular injury in salt-sensitive hypertensive rats independently of blood glucose and blood pressure. Cardiovasc Diabetol. 2014; 13:157. PMID: 25471116.
Article9. Ihara M, Asanuma H, Yamazaki S, Kato H, Asano Y, Shinozaki Y, Mori H, Minamino T, Asakura M, Sugimachi M, Mochizuki N, Kitakaze M. An interaction between glucagon-like peptide-1 and adenosine contributes to cardioprotection of a dipeptidyl peptidase 4 inhibitor from myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2015; 308:H1287–H1297. PMID: 25747753.
Article10. Rodríguez-Mañas L, Angulo J, Vallejo S, Peiró C, Sánchez-Ferrer A, Cercas E, López-Dóriga P, Sánchez-Ferrer CF. Early and intermediate Amadori glycosylation adducts, oxidative stress, and endothelial dysfunction in the streptozotocin-induced diabetic rats vasculature. Diabetologia. 2003; 46:556–566. PMID: 12739028.
Article11. Gutch M, Joshi A, Kumar S, Agarwal A, Pahan RK, Razi SM. Gemigliptin: newer promising gliptin for type 2 diabetes mellitus. Indian J Endocrinol Metab. 2017; 21:898–902. PMID: 29285456.
Article12. Park SE, Lee BW, Kim JH, Lee WJ, Cho JH, Jung CH, Lee SH, Suh S, Hur GC, Kim SH, Jang YH, Park CY. Effect of gemigliptin on glycaemic variability in patients with type 2 diabetes (STABLE study). Diabetes Obes Metab. 2017; 19:892–896. PMID: 28058753.
Article13. Incalza MA, D'Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018; 100:1–19. PMID: 28579545.
Article14. Craige SM, Kant S, Keaney JF Jr. Reactive oxygen species in endothelial function - from disease to adaptation. Circ J. 2015; 79:1145–1155. PMID: 25986771.15. Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012; 32:4812–4820. PMID: 22492036.
Article16. Silva BR, Pernomian L, Bendhack LM. Contribution of oxidative stress to endothelial dysfunction in hypertension. Front Physiol. 2012; 3:441. PMID: 23227009.
Article17. Salheen SM, Panchapakesan U, Pollock CA, Woodman OL. The DPP-4 inhibitor linagliptin and the GLP-1 receptor agonist exendin-4 improve endothelium-dependent relaxation of rat mesenteric arteries in the presence of high glucose. Pharmacol Res. 2015; 94:26–33. PMID: 25697548.
Article18. Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, Xu G, Pu Y, Zhu Z, Xu A, Lam KS, Chen ZY, Ng CF, Yao X, Huang Y. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension. 2012; 60:833–841. PMID: 22868389.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of gemigliptin on cardiac ischemia/reperfusion and spontaneous hypertensive rat models
- Gemigliptin: An Update of Its Clinical Use in the Management of Type 2 Diabetes Mellitus
- Effect of Dipeptidyl Peptidase-4 Inhibitors on Cardiovascular Outcome
- Dipeptidyl Peptidase-4 Inhibitor
- Characterization of ETB Receptor-mediated Relaxation in Precontracted Mesenteric Artery from Streptozotocin-induced Diabetic Rats