Anat Cell Biol.
2015 Sep;48(3):177-187. 10.5115/acb.2015.48.3.177.
Regional differences in the density of Langerhans cells, CD8-positive T lymphocytes and CD68-positive macrophages: a preliminary study using elderly donated cadavers
- Affiliations
-
- 1Department of Anatomy, Tokyo Dental College, Tokyo, Japan. yamamotomasahito@tdc.ac.jp
- 2Department of Urology, Kobe University Graduate School of Medicine, Kobe, Japan.
- 3Division of Internal Medicine, Iwamizawa Kojin-kai Hospital, Iwamizawa, Japan.
- KMID: 2424820
- DOI: http://doi.org/10.5115/acb.2015.48.3.177
Abstract
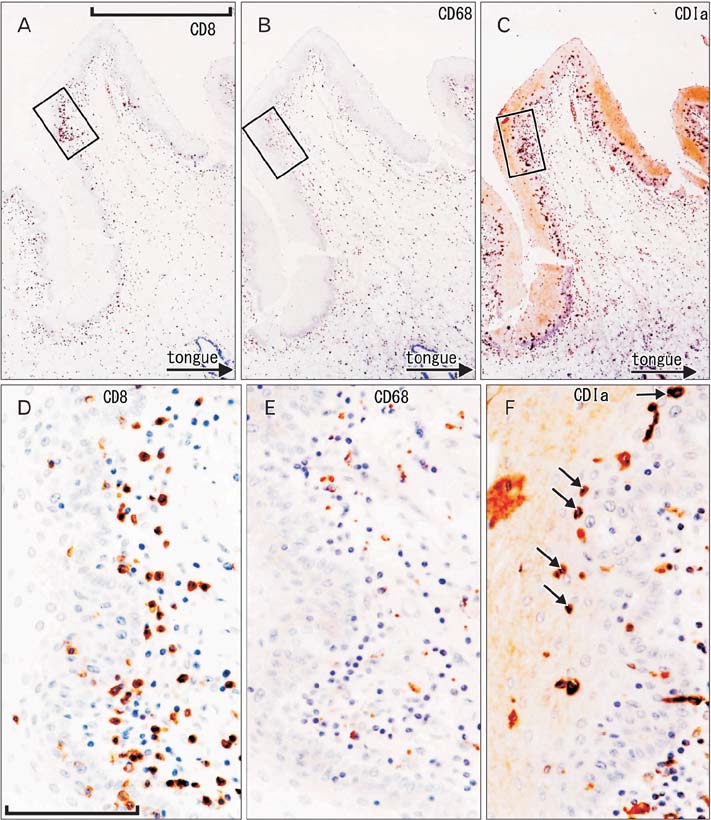
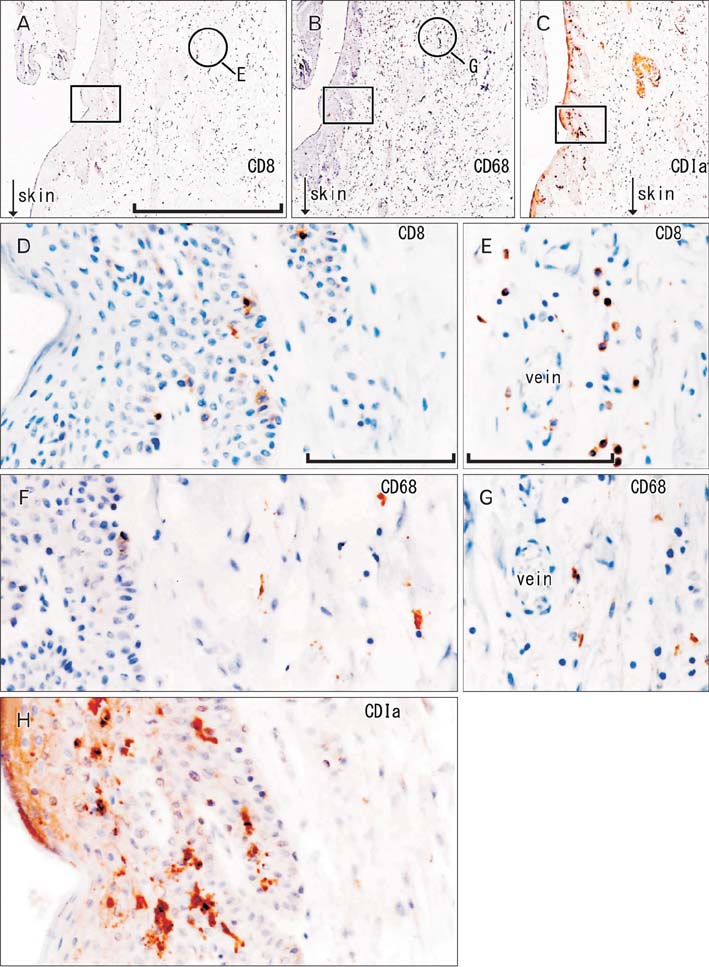
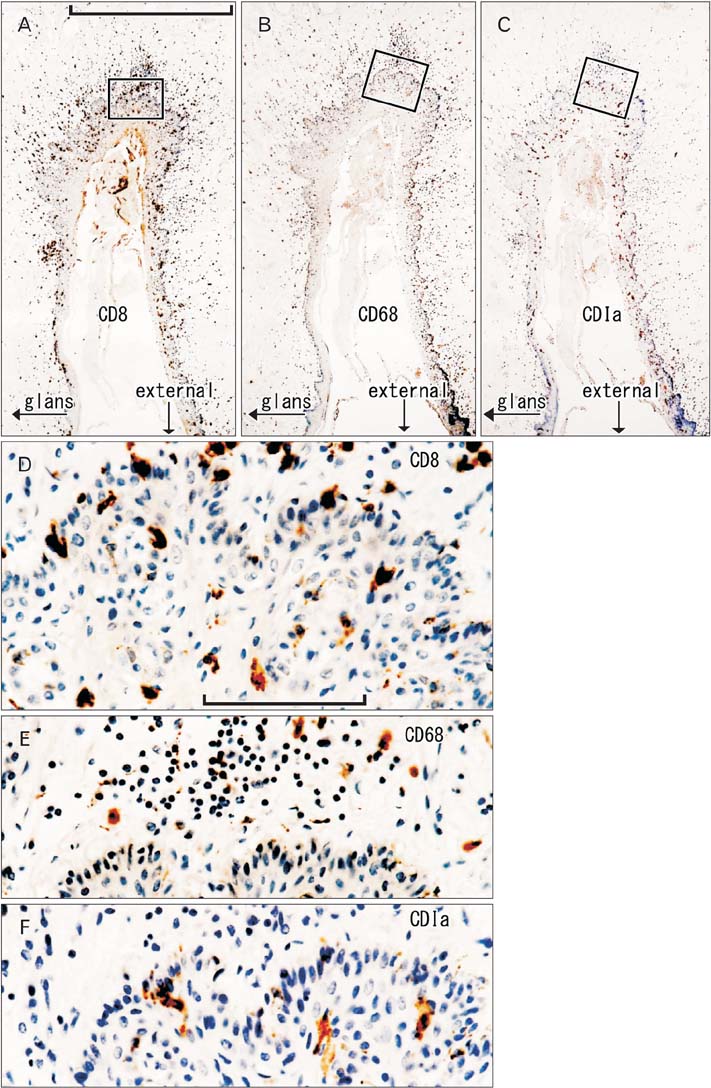
- To provide a better understanding of the local immune system in the face and external genitalia, i.e., the oral floor, lower lip, palpebral conjunctiva, anus and penis, we examined the distribution and density of CD1a-positve Langerhans cells, CD8-positive suppressor T lymphocytes and CD68-positive macrophages using specimens from 8 male elderly cadavers. The density of Langerhans cells showed an individual difference of more than (or almost) 10-fold in the lip (oral floor). In the oral floor, Langerhans cells were often spherical. Submucosal or subcutaneous suppressor lymphocytes, especially rich in the oral floor and penile skin, migrated into the epithelium at 4 sites, except for the anus. In the conjunctiva, macrophage migration into the epithelium was seen in all 8 specimens. The density of suppressor lymphocytes showed a significant correlation between the oral floor and the lip (r=0.78). In contrast, the anal and penile skins showed no positive correlation in the density of all three types of immunoreactive cells examined. Overall, irrespective of the wide individual differences, the oral floor and conjunctiva seemed to be characterized by a rich content of all three cell types, whereas the penile skin was characterized by an abundance of suppressor lymphocytes. Based on the tables, as mean value, the relative abundance of three different cell types were as follows; CD1a-positive Langerhans cells (anus), CD8-positive lymphocytes (penis), and CD68-positive macrophages (lip). The present observations suggest that the local immune response is highly site-dependent, with a tendency for tolerance rather than rejection.
Keyword
MeSH Terms
Figure
Reference
-
1. Cruchley AT, Williams DM, Farthing PM, Lesch CA, Squier CA. Regional variation in Langerhans cell distribution and density in normal human oral mucosa determined using monoclonal antibodies against CD1, HLADR, HLADQ and HLADP. J Oral Pathol Med. 1989; 18:510–516.2. Linde N, Lederle W, Depner S, van Rooijen N, Gutschalk CM, Mueller MM. Vascular endothelial growth factor-induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J Pathol. 2012; 227:17–28.3. Muthupalani S, Ge Z, Feng Y, Rickman B, Mobley M, McCabe A, Van Rooijen N, Fox JG. Systemic macrophage depletion inhibits Helicobacter bilis-induced proinflammatory cytokine-mediated typhlocolitis and impairs bacterial colonization dynamics in a BALB/c Rag2-/- mouse model of inflammatory bowel disease. Infect Immun. 2012; 80:4388–4397.4. Zhou D, Chen YT, Chen F, Gallup M, Vijmasi T, Bahrami AF, Noble LB, van Rooijen N, McNamara NA. Critical involvement of macrophage infiltration in the development of Sjogren's syndrome-associated dry eye. Am J Pathol. 2012; 181:753–760.5. Lam RS, O'Brien-Simpson NM, Lenzo JC, Holden JA, Brammar GC, Walsh KA, McNaughtan JE, Rowler DK, Van Rooijen N, Reynolds EC. Macrophage depletion abates Porphyromonas gingivalis-induced alveolar bone resorption in mice. J Immunol. 2014; 193:2349–2362.6. Schneider C, Nobs SP, Heer AK, Kurrer M, Klinke G, van Rooijen N, Vogel J, Kopf M. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog. 2014; 10:e1004053.7. Zaslona Z, Przybranowski S, Wilke C, van Rooijen N, Teitz-Tennenbaum S, Osterholzer JJ, Wilkinson JE, Moore BB, Peters-Golden M. Resident alveolar macrophages suppress, whereas recruited monocytes promote, allergic lung inflammation in murine models of asthma. J Immunol. 2014; 193:4245–4253.8. Hwang SE, Kim JH, Yu HC, Murakami G, Cho BH. Lymphocyte subpopulations in the liver, spleen, intestines, and mesenteric nodes: an immunohistochemical study using human fetuses at 15-16 weeks. Anat Rec (Hoboken). 2014; 297:1478–1489.9. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. N Engl J Med. 1991; 324:1–8.10. Jaitley S, Saraswathi T. Pathophysiology of Langerhans cells. J Oral Maxillofac Pathol. 2012; 16:239–244.11. de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MA, de Gruijl T, Piguet V, van Kooyk Y, Geijtenbeek TB. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007; 13:367–371.12. Morelli AE, Ronchetti RD, Secchi AD, Cufré MA, Paredes A, Fainboim L. Assessment by planimetry of Langerhans' cell density in penile epithelium with human papillomavirus infection: changes observed after topical treatment. J Urol. 1992; 147:1268–1273.13. Balat A, Karakök M, Güler E, Uçaner N, Kibar Y. Local defense systems in the prepuce. Scand J Urol Nephrol. 2008; 42:63–65.14. Qin Q, Zheng XY, Wang YY, Shen HF, Sun F, Ding W. Langerhans' cell density and degree of keratinization in foreskins of Chinese preschool boys and adults. Int Urol Nephrol. 2009; 41:747–753.15. Price LB, Liu CM, Johnson KE, Aziz M, Lau MK, Bowers J, Ravel J, Keim PS, Serwadda D, Wawer MJ, Gray RH. The effects of circumcision on the penis microbiome. PLoS One. 2010; 5:e8422.16. Polak ME, Newell L, Taraban VY, Pickard C, Healy E, Friedmann PS, Al-Shamkhani A, Ardern-Jones MR. CD70-CD27 interaction augments CD8+ T-cell activation by human epidermal Langerhans cells. J Invest Dermatol. 2012; 132:1636–1644.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- TNFalpha Expression in the Paraffin-embedded Tissues of Leprosy
- The Expression and Relationship of CD68-Tumor-Associated Macrophages and Microvascular Density With the Prognosis of Patients With Laryngeal Squamous Cell Carcinoma
- Increased Immunoendocrine Cells in Intestinal Mucosa of Postinfectious Irritable Bowel Syndrome Patients 3 Years after Acute Shigella Infection: An Observation in a Small Case Control Study
- Macrophage density in pharyngeal and laryngeal muscles greatly exceeds that in other striated muscles: an immunohistochemical study using elderly human cadavers
- Distribution of MHC class II Positive Cells and Macrophages in the Iris and Ciliary Body in Endotoxin Induced Uveitis