Anat Cell Biol.
2016 Sep;49(3):177-183. 10.5115/acb.2016.49.3.177.
Macrophage density in pharyngeal and laryngeal muscles greatly exceeds that in other striated muscles: an immunohistochemical study using elderly human cadavers
- Affiliations
-
- 1Department of Anatomy, Tokyo Dental College, Tokyo, Japan. yamamotomasahito@tdc.ac.jp
- 2Department of Otorhinolaryngology, Tohoku University School of Medicine, Sendai, Japan.
- 3Division of Internal Medicine, Iwamizawa Asuka Hospital, Iwamizawa, Japan.
- KMID: 2353342
- DOI: http://doi.org/10.5115/acb.2016.49.3.177
Abstract
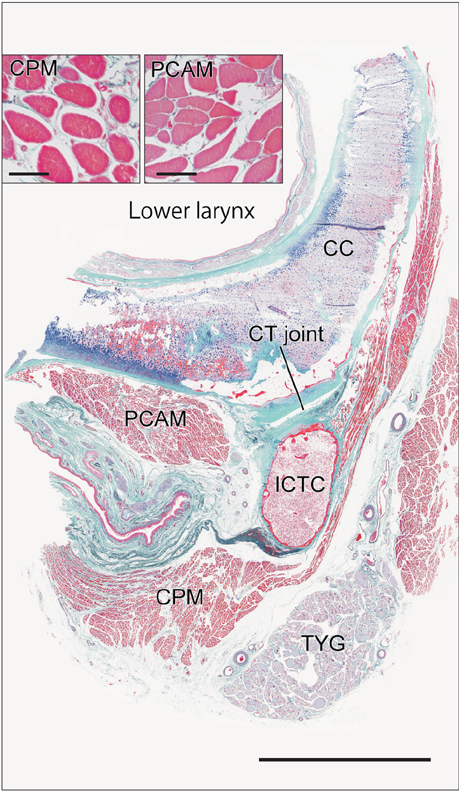
- Macrophages play an important role in aging-related muscle atrophy (i.e., sarcopenia). We examined macrophage density in six striated muscles (cricopharyngeus muscle, posterior cricoarytenoideus muscle, genioglossus muscle, masseter muscle, infraspinatus muscle, and external anal sphincter). We examined 14 donated male cadavers and utilized CD68 immunohistochemistry to clarify macrophage density in muscles. The numbers of macrophages per striated muscle fiber in the larynx and pharynx (0.34 and 0.31) were 5-6 times greater than those in the tongue, shoulder, and anus (0.05-0.07) with high statistical significance. Thick muscle fibers over 80 µm in diameter were seen in the pharynx, larynx, and anal sphincter of two limited specimens. Conversely, in the other sites or specimens, muscle fibers were thinner than 50 µm. We did not find any multinuclear muscle cells suggestive of regeneration. At the beginning of the study, we suspected that mucosal macrophages might have invaded into the muscle layer of the larynx and pharynx, but we found no evidence of inflammation in the mucosa. Likewise, the internal anal sphincter (a smooth muscle layer near the mucosa) usually contained fewer macrophages than the external sphincter. The present result suggest that, in elderly men, thinning and death of striated muscle fibers occur more frequently in the larynx and pharynx than in other parts of the body.
Keyword
MeSH Terms
Figure
Reference
-
1. Ryan M, Butler-Browne G, Erzen I, Mouly V, Thornell LE, Wernig A, Ohlendieck K. Persistent expression of the alpha1S-dihydropyridine receptor in aged human skeletal muscle: implications for the excitation-contraction uncoupling hypothesis of sarcopenia. Int J Mol Med. 2003; 11:425–434.2. Pietrangelo T, Puglielli C, Mancinelli R, Beccafico S, Fanò G, Fulle S. Molecular basis of the myogenic profile of aged human skeletal muscle satellite cells during differentiation. Exp Gerontol. 2009; 44:523–531.3. Cruse JP, Edwards DA, Smith JF, Wyllie JH. The pathology of a cricopharyngeal dysphagia. Histopathology. 1979; 3:223–232.4. Mu L, Sobotka S, Chen J, Su H, Sanders I, Adler CH, Shill HA, Caviness JN, Samanta JE, Beach TG. Arizona Parkinson's Disease Consortium. Altered pharyngeal muscles in Parkinson disease. J Neuropathol Exp Neurol. 2012; 71:520–530.5. Yeung D, Kharidia R, Brown SC, Górecki DC. Enhanced expression of the P2X4 receptor in Duchenne muscular dystrophy correlates with macrophage invasion. Neurobiol Dis. 2004; 15:212–220.6. Desguerre I, Mayer M, Leturcq F, Barbet JP, Gherardi RK, Christov C. Endomysial fibrosis in Duchenne muscular dystrophy: a marker of poor outcome associated with macrophage alternative activation. J Neuropathol Exp Neurol. 2009; 68:762–773.7. Kobayashi K, Izawa T, Kuwamura M, Yamate J. The distribution and characterization of skeletal muscle lesions in dysferlin-deficient SJL and A/J mice. Exp Toxicol Pathol. 2010; 62:509–517.8. Gumucio JP, Davis ME, Bradley JR, Stafford PL, Schiffman CJ, Lynch EB, Claflin DR, Bedi A, Mendias CL. Rotator cuff tear reduces muscle fiber specific force production and induces macrophage accumulation and autophagy. J Orthop Res. 2012; 30:1963–1970.9. Gumucio J, Flood M, Harning J, Phan A, Roche S, Lynch E, Bedi A, Mendias C. T lymphocytes are not required for the development of fatty degeneration after rotator cuff tear. Bone Joint Res. 2014; 3:262–272.10. Gumucio JP, Korn MA, Saripalli AL, Flood MD, Phan AC, Roche SM, Lynch EB, Claflin DR, Bedi A, Mendias CL. Aging-associated exacerbation in fatty degeneration and infiltration after rotator cuff tear. J Shoulder Elbow Surg. 2014; 23:99–108.11. Evans WJ. Effects of exercise on senescent muscle. Clin Orthop Relat Res. 2002; 403 Suppl. S211–S220.12. Siriett V, Salerno MS, Berry C, Nicholas G, Bower R, Kambadur R, Sharma M. Antagonism of myostatin enhances muscle regeneration during sarcopenia. Mol Ther. 2007; 15:1463–1470.13. Colombo E, Bedogni F, Lorenzetti I, Landsberger N, Previtali SC, Farina C. Autocrine and immune cell-derived BDNF in human skeletal muscle: implications for myogenesis and tissue regeneration. J Pathol. 2013; 231:190–198.14. Lang IM, Shaker R. An overview of the upper esophageal sphincter. Curr Gastroenterol Rep. 2000; 2:185–190.15. Sivarao DV, Goyal RK. Functional anatomy and physiology of the upper esophageal sphincter. Am J Med. 2000; 108:Suppl 4a. 27S–37S.16. Ertekin C, Aydogdu I. Electromyography of human cricopharyngeal muscle of the upper esophageal sphincter. Muscle Nerve. 2002; 26:729–739.17. Ishiyama G, Hinata N, Kinugasa Y, Murakami G, Fujimiya M. Nerves supplying the internal anal sphincter: an immunohistochemical study using donated elderly cadavers. Surg Radiol Anat. 2014; 36:1033–1042.18. Kim JH, Kinugasa Y, Yu HC, Murakami G, Abe S, Cho BH. Lack of striated muscle fibers in the longitudinal anal muscle of elderly Japanese: a histological study using cadaveric specimens. Int J Colorectal Dis. 2015; 30:43–49.19. Kawamoto A, Honkura Y, Suzuki R, Abe H, Abe S, Murakami G, Katori Y. Cricothyroid articulation in elderly Japanese with special reference to morphology of the synovial and capsular tissues. J Voice. 2016; 30:538–548.20. Serikawa M, Yamamoto M, Kawamoto A, Katori Y, Kinoshita H, Matsunaga S, Abe S. The cricothyroid joint in elderly Japanese individuals. Anat Sci Int. 2016; 91:250–257.21. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. N Engl J Med. 1991; 324:1–8.22. Cook IJ, Blumbergs P, Cash K, Jamieson GG, Shearman DJ. Structural abnormalities of the cricopharyngeus muscle in patients with pharyngeal (Zenker's) diverticulum. J Gastroenterol Hepatol. 1992; 7:556–562.23. Reimann J, Schnell S, Schwartz S, Kappes-Horn K, Dodel R, Bacher M. Macrophage migration inhibitory factor in normal human skeletal muscle and inflammatory myopathies. J Neuropathol Exp Neurol. 2010; 69:654–662.24. Pereira LJ, Gavião MB, Bonjardim LR, Castelo PM, van der Bilt A. Muscle thickness, bite force, and craniofacial dimensions in adolescents with signs and symptoms of temporomandibular dysfunction. Eur J Orthod. 2007; 29:72–78.25. Ozaki M, Kaneko S, Soma K. Masseter muscular weakness affects temporomandibular synovitis induced by jaw opening in growing rats. Angle Orthod. 2008; 78:819–825.26. Sano R, Tanaka E, Korfage JA, Langenbach GE, Kawai N, van Eijden TM, Tanne K. Heterogeneity of fiber characteristics in the rat masseter and digastric muscles. J Anat. 2007; 211:464–470.27. Bar-Shai M, Carmeli E, Coleman R, Rozen N, Perek S, Fuchs D, Reznick AZ. The effect of hindlimb immobilization on acid phosphatase, metalloproteinases and nuclear factor-kappaB in muscles of young and old rats. Mech Ageing Dev. 2005; 126:289–297.28. Wehling-Henricks M, Jordan MC, Gotoh T, Grody WW, Roos KP, Tidball JG. Arginine metabolism by macrophages promotes cardiac and muscle fibrosis in mdx muscular dystrophy. PLoS One. 2010; 5:e10763.29. Villalta SA, Rinaldi C, Deng B, Liu G, Fedor B, Tidball JG. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Hum Mol Genet. 2011; 20:790–805.30. Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol. 2012; 189:3669–3680.31. Wang Y, Wehling-Henricks M, Samengo G, Tidball JG. Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging Cell. 2015; 14:678–688.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fetal anatomy of the upper pharyngeal muscles with special reference to the nerve supply: is it an enteric plexus or simply an intramuscular nerve?
- Normal Swallowing Mechanism on Neurophysiogical Basis
- Histochemical Study of Musculature of the Human Upper Esophageal Sphincter
- Anatomical variations of the stylopharyngeus and superior constrictors in relation to their function
- Neck Discomfort after Thyroidectomy