Yonsei Med J.
2010 Jan;51(1):45-51. 10.3349/ymj.2010.51.1.45.
Increased Immunoendocrine Cells in Intestinal Mucosa of Postinfectious Irritable Bowel Syndrome Patients 3 Years after Acute Shigella Infection: An Observation in a Small Case Control Study
- Affiliations
-
- 1Department of Internal Medicine, Yonsei Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea. HJPARK21@yuhs.ac
- KMID: 1779605
- DOI: http://doi.org/10.3349/ymj.2010.51.1.45
Abstract
- PURPOSE
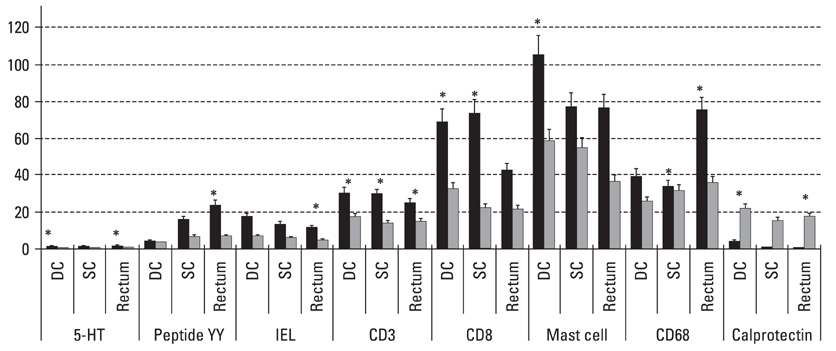
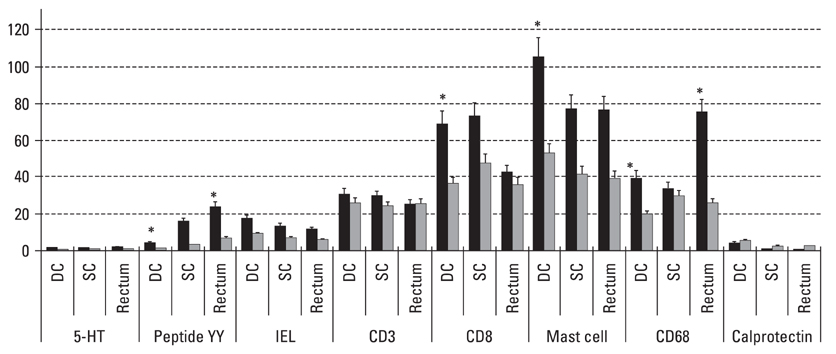
Postinfectiously irritable bowel syndrome (PI-IBS) develops in 3-30% of individuals with bacterial gastroenteritis. Recent studies demonstrated increases in inflammatory components in gut mucosa of PI-IBS patients even after complete resolution of infection. We aimed to investigate histological changes in colon and rectum of PI-IBS subjects after long term period of infection. MATERIALS AND METHODS: We recruited PI-IBS subjects who had been diagnosed IBS after complete resolution of enteritis caused by shigellosis outbreak 3 years earlier. We compared unmatched four groups, PI-IBS (n = 4), non PI-IBS (n = 7), D-IBS (n = 7, diarrhea predominant type) and healthy controls (n = 10). All of them underwent colonoscopic biopsy at three areas, including descending colon (DC), sigmoid colon (SC) and rectum, which were assessed for 5-hydroxytryptamine (5-HT)/peptide YY (PYY)-containing enterochromaffin (EC) cell, intraepithelial (IEL) and lamina propria T lymphocyte (CD3), CD8 lymphocytes, mast cells and CD68/calprotectin+ macrophages. RESULTS: All subjects had no structural or gross abnormalities at colonoscopy. In PI-IBS, 5-HT containing EC cells, PYY containing EC cells, IELs, CD3 lymphocytes, CD8 lymphocytes, mast cells, and CD68 + macrophages were increased compared to control (p < 0.05). In D-IBS, PYY containing EC cells, IELs, and CD3 lymphocytes were increased compared to control (p < 0.05). In PI-IBS, 5-HT containing EC cells tended to increase and PYY containing EC cells, CD8 lymphocytes, mast cells, and CD68+ macrophages were increased compared to non PI-IBS (p < 0.05). Calprotectin + marcrophages were decreased in PI-IBS, non PI-IBS and IBS compared to control. CONCLUSION: The immunoendocrine cells were sporadically increased in PI-IBS, non PI-IBS and D-IBS compared with control. Our findings in a very small number of patients suggest that mucosal inflammation may play a role in long-term PI-IBS, and that other sub-groups of IBS and larger scale studies are needed to confirm this observation.
Keyword
MeSH Terms
-
Adult
Antigens, CD/metabolism
Antigens, Differentiation, Myelomonocytic/metabolism
CD8-Positive T-Lymphocytes/cytology
Case-Control Studies
Colon, Descending/pathology
Colon, Sigmoid/pathology
Colonoscopy
Dysentery, Bacillary/*complications
Enterochromaffin Cells/cytology
Female
Humans
Immunohistochemistry
Intestinal Mucosa/*pathology
Irritable Bowel Syndrome/metabolism/*pathology
Macrophages/cytology
Male
Mast Cells/cytology
Peptide YY/metabolism
Rectum/pathology
Serotonin/metabolism
Figure
Reference
-
1. Gwee KA, Leong YL, Graham C, McKendrick MW, Collins SM, Walters SJ, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999. 44:400–406.
Article2. Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003. 125:1651–1659.
Article3. Gwee KA, Graham JC, McKendrick MW, Collins SM, Marshall JS, Walters SJ, et al. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet. 1996. 347:150–153.
Article4. McKendrick MW, Read NW. Irritable bowel syndrome-post salmonella infection. J Infect. 1994. 29:1–3.
Article5. Neal KR, Barker L, Spiller RC. Prognosis in post-infective irritable bowel syndrome: a six-year follow-up study. Gut. 2002. 51:410–413.
Article6. Neak KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. BMJ. 1997. 314:779–782.7. Rodríguez LA, Ruigómez A. Increased risk of irritable bowel syndrome after bacterial gastroenteritis: cohort study. BMJ. 1999. 318:565–566.8. Hiatt RB, Katz J. Mast cells in inflammatory conditions of the gastrointestinal tract. Am J Gastroenterol. 1962. 37:541–545.9. Weston AP, Biddle WL, Bhatia PS, Miner PB Jr. Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig Dis Sci. 1993. 38:1590–1595.
Article10. O'Sullivan M, Clayton N, Breslin NP, Harman I, Bountra C, McLaren A, et al. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000. 12:449–457.11. Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004. 126:693–702.
Article12. Park CH, Joo YE, Choi SK, Rew JS, Kim SJ, Lee MC. Activated mast cells infiltrate in close proximity to enteric nerves in diarrheapredominant irritable bowel syndrome. J Korean Med Sci. 2003. 18:204–210.
Article13. Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002. 122:1778–1783.
Article14. Kim HS, Kim MS, Ji SW, Park H. The development of irritable bowel syndrome after Shigella infection: 3 year follow-up study. Korean J Gastroenterol. 2006. 47:300–305.15. Pardi DS, Ramnath VR, Loftus EV Jr, Tremaine WJ, Sandborn WJ. Lymphocytic colitis: clinical features, treatment, and outcomes. Am J Gastroenterol. 2002. 97:2829–2833.
Article16. Veress B, Löberg R, Bergman L. Microscopic colitis syndrome. Gut. 1995. 36:880–886.17. Limsui D, Pardi DS, Camilleri M, Loftus EV Jr, Kammer PP, Tremaine WJ, et al. Symptomatic overlap between irritable bowel syndrome and microscopic colitis. Inflamm Bowel Dis. 2007. 13:175–181.18. Ji S, Park H, Lee D, Song YK, Choi JP, Lee SI. Post-infectious irritable bowel syndrome in patients with Shigella infection. J Gastroenterol Hepatol. 2005. 20:381–386.
Article19. Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003. 52:439–451.20. Kucharzik T, Maaser C, Lüering A, Kagnoff M, Mayer L, Targan S, et al. Recent understanding of IBD pathogenesis: implications for future therapies. Inflamm Bowel Dis. 2006. 12:1068–1083.21. Tack J, Demedts I, Dehondt G, Caenepeel P, Fischler B, Zandecki M, et al. Clinical and pathophysiological characteristics of acute-onset functional dyspepsia. Gastroenterology. 2002. 122:1738–1747.22. Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003. 124:1662–1671.23. Bardhan PK, Salam MA, Molla AM. Gastric emptying of liquid in children suffering from acute rotaviral gastroenteritis. Gut. 1992. 33:26–29.
Article24. Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994. 107:271–293.25. Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000. 47:804–811.
Article26. Dunlop SP, Jenkins DJ, Spiller RC. Distictive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol. 2003. 98:1578–1583.27. Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000. 95:2698–2709.28. Tonini M. 5-Hydroxytryptamine effects in the gut: the 3,4, and 7 receptors. Neurogastroenterol Motil. 2005. 17:637–642.29. Imamura M. Effects of surgical manipulation of the intestine on peptide YY and its physiology. Peptides. 2002. 23:403–407.30. Imamura M, Nakajima H, Mikami Y, Yamauchi H. Morphological and immunohistochemical changes in intestinal mucosa and PYY release following total colectomy with ileal pouch-anal anastomosis in dogs. Dig Dis Sci. 1999. 44:1000–1007.31. Kong W, McConaloque K, Khitin LM, Hollenberg MD, Payan DG, Böhm SK, et al. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc Natl Acad Sci U S A. 1997. 94:8884–8889.
Article32. Li Y, Yio XY, Mayer L. Human intestinal epithelial cell-induced CD8+ T cell activation is mediated through CD8 and the activation of CD8-associated p56lck. J Exp Med. 1995. 182:1079–1088.
Article33. Bjerke K, Halstensen TS, Jahnsen F, Pulford K, Brandtzaeg P. Distribution of macrophages and granulocytes expressing L1 protein(calprotectin) in human Peyer's patches compared with normal ileal lamina propria and mesenteric lymph nodes. Gut. 1993. 34:1357–1363.34. Rugtveit J, Brandtzaeg P, Halstensen TS, Fausa O, Scott H. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut. 1994. 35:669–674.
Article35. Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008. 214:161–178.36. Hausmann M, Bataille F, Spoettl T, Schreiter K, Falk W, Schoelmerich J, et al. Physiological role of macrophage inflammatory protein-3alpha induction during maturation of intestinal macrophages. J Immunol. 2005. 175:1389–1398.
Article37. Motomura Y, Ghia JE, Wang H, Akiho H, El-Sharkawy RT, Collins M, et al. Enterochromaffin cell and 5-hydroxytryptamine responses to the same infectious agent differ in Th1 and Th2 dominant environments. Gut. 2008. 57:475–481.
Article38. Törnblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002. 123:1972–1979.
Article39. Chadwick VS, Chen W, Shu D, Paulus B, Bethwait P, Tie A, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002. 122:1778–1783.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mast Cell May Be the Master Key to Solve the Mystery of Pathogenesis of Irritable Bowel Syndrome
- Alteration of Fecal Microbiota in Patients With Postinfectious Irritable Bowel Syndrome
- The Relationship between Small-Intestinal Bacterial Overgrowth and Intestinal Permeability in Patients with Irritable Bowel Syndrome
- The Role of Small Intestinal Bacterial Overgrowth in the Pathophysiology of Irritable Bowel Syndrome
- The Development of Irritable Bowel Syndrome after Shigella Infection: 3 Year Follow-up Study