Immune Netw.
2012 Aug;12(4):155-164.
Affinity Maturation of an Epidermal Growth Factor Receptor Targeting Human Monoclonal Antibody ER414 by CDR Mutation
- Affiliations
-
- 1Antibody Engineering Lab., Green Cross Research Center, Green Cross Corp., Yongin 446-770, Korea. sehokim@greencross.com
Abstract
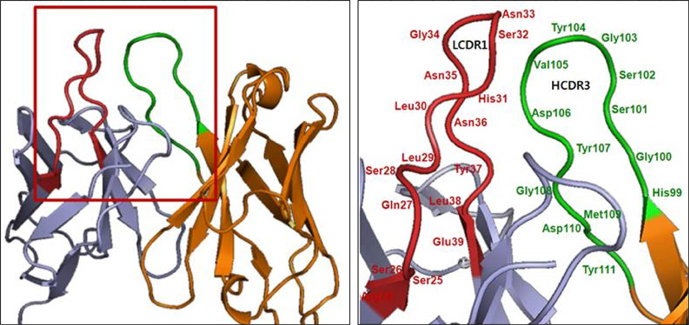
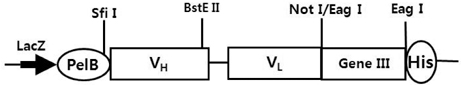
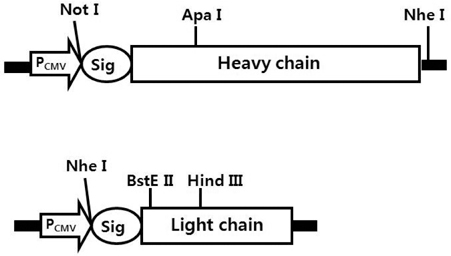
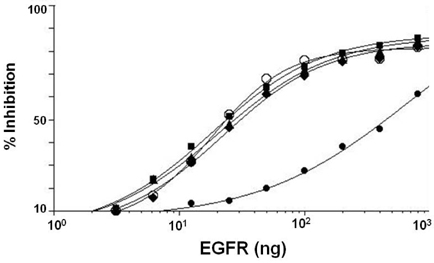
- It is well established that blocking the interaction of EGFR with growth factors leads to the arrest of tumor growth, resulting in tumor cell death. ER414 is a human monoclonal antibody (mAb) derived by guided selection of the mouse mAb A13. The ER414 exhibited a ~17-fold lower affinity and, as a result, lower efficacy of inhibition of the EGF-mediated tyrosine phosphorylation of EGFR when compared with mAb A13 and cetuximab. We performed a stepwise in vitro affinity maturation to improve the affinity of ER414. We obtained a 3D model of ER414 to identify the amino acids in the CDRs that needed to be mutated. Clones were selected from the phage library with randomized amino acids in the CDRs and substitution of amino acids in the HCDR3 and LCDR1 of ER414 led to improved affinity. A clone, H3-14, with a ~20-fold increased affinity, was selected from the HCDR3 randomized library. Then three clones, ER2, ER78 and ER79, were selected from the LCDR1 randomized library based on the H3-14 but did not show further increased affinities compared to that of H3-14. Of the three, ER2 was chosen for further characterization due to its better expression than others. We successfully performed affinity maturation of ER414 and obtained antibodies with a similar affinity as cetuximab. And antibody from an affinity maturation inhibits the EGF-mediated tyrosine phosphorylation of EGFR in a manner similar to cetuximab.
Keyword
MeSH Terms
-
Amino Acids
Animals
Antibodies
Antibodies, Monoclonal, Humanized
Bacteriophages
Cell Death
Cetuximab
Clone Cells
Deoxycytidine
Epidermal Growth Factor
Humans
Intercellular Signaling Peptides and Proteins
Mice
Phosphorylation
Receptor, Epidermal Growth Factor
Tyrosine
Amino Acids
Antibodies
Antibodies, Monoclonal, Humanized
Deoxycytidine
Epidermal Growth Factor
Intercellular Signaling Peptides and Proteins
Receptor, Epidermal Growth Factor
Tyrosine
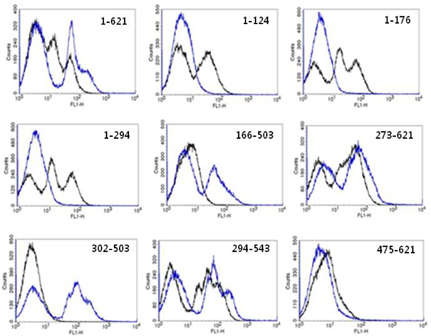
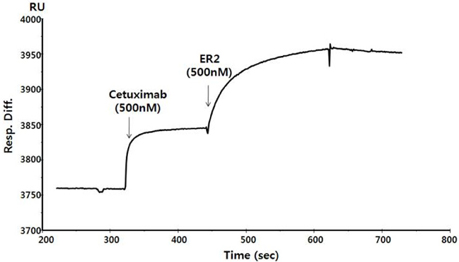
Figure
Reference
-
1. Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987. 56:881–914.
Article2. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001. 2:127–137.
Article3. Laskin JJ, Sandler AB. Epidermal growth factor receptor: a promising target in solid tumours. Cancer Treat Rev. 2004. 30:1–17.
Article4. Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer. 2002. 94:1593–1611.
Article5. Capdevila J, Elez E, Macarulla T, Ramos FJ, Ruiz-Echarri M, Tabernero J. Anti-epidermal growth factor receptor monoclonal antibodies in cancer treatment. Cancer Treat Rev. 2009. 35:354–363.
Article6. Brabender J, Danenberg KD, Metzger R, Schneider PM, Park J, Salonga D, Hölscher AH, Danenberg PV. Epidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer Is correlated with survival. Clin Cancer Res. 2001. 7:1850–1855.7. Baselga J. Targeting the epidermal growth factor receptor with tyrosine kinase inhibitors: small molecules, big hopes. J Clin Oncol. 2002. 20:2217–2219.
Article8. Thomas SM, Grandis JR. Pharmacokinetic and pharmacodynamic properties of EGFR inhibitors under clinical investigation. Cancer Treat Rev. 2004. 30:255–268.
Article9. Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004. 59:2 Suppl. 21–26.
Article10. Chang KH, Kim MS, Hong GW, Shin YN, Kim SH. Conversion of a murine monoclonal antibody A13 targeting epidermal growth factor receptor to a human monoclonal antibody by guided selection. Exp Mol Med. 2012. 44:52–59.
Article11. Kim SH, Park SY. Selection and characterization of human antibodies against hepatitis B virus surface antigen (HBsAg) by phage-display. Hybrid Hybridomics. 2002. 21:385–392.
Article12. Kim SH, Chun JH, Park SY. Characterization of monoclonal antibodies against carcinoembryonic antigen (CEA) and expression in E. coli. Hybridoma. 2001. 20:265–272.
Article13. Kim SH, Song SH, Kim YJ, Park SY. Expression and characterization of a recombinant Fab fragment derived from an anti-human alpha-fetoprotein monoclonal antibody. Mol Cells. 2001. 11:158–163.14. Shin YW, Ryoo KH, Hong KW, Chang KH, Choi JS, So M, Kim PK, Park JY, Bong KT, Kim SH. Human monoclonal antibody against Hepatitis B virus surface antigen (HBsAg). Antiviral Res. 2007. 75:113–120.
Article15. Urlaub G, Mitchell PJ, Kas E, Chasin LA, Funanage VL, Myoda TT, Hamlin J. Effect of gamma rays at the dihydrofolate reductase locus: deletions and inversions. Somat Cell Mol Genet. 1986. 12:555–566.
Article16. Hong KW, Kim CG, Lee SH, Chang KH, Shin YW, Ryoo KH, Kim SH, Kim YS. A novel anti-EGFR monoclonal antibody inhibiting tumor cell growth by recognizing different epitopes from cetuximab. J Biotechnol. 2010. 145:84–91.
Article17. Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002. 62:4132–4141.18. Kim YS, Bhandari R, Cochran JR, Kuriyan J, Wittrup KD. Directed evolution of the epidermal growth factor receptor extracellular domain for expression in yeast. Proteins. 2006. 62:1026–1035.
Article19. Kim MS, Lee SH, Song MY, Yoo TH, Lee BK, Kim YS. Comparative analyses of complex formation and binding sites between human tumor necrosis factor-alpha and its three antagonists elucidate their different neutralizing mechanisms. J Mol Biol. 2007. 374:1374–1388.
Article20. Song MY, Park SK, Kim CS, Yoo TH, Kim B, Kim MS, Kim YS, Kwag WJ, Lee BK, Baek K. Characterization of a novel anti-human TNF-alpha murine monoclonal antibody with high binding affinity and neutralizing activity. Exp Mol Med. 2008. 40:35–42.
Article21. Cochran JR, Kim YS, Olsen MJ, Bhandari R, Wittrup KD. Domain-level antibody epitope mapping through yeast surface display of epidermal growth factor receptor fragments. J Immunol Methods. 2004. 287:147–158.
Article22. Burgess AW. EGFR family: structure physiology signalling and therapeutic targets. Growth Factors. 2008. 26:263–274.
Article23. Chothia C, Lesk AM. Canonical structures for the hypervariable regions of immunoglobulins. J Mol Biol. 1987. 196:901–917.
Article24. Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005. 7:301–311.
Article25. Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, Alpaugh RK, Marks JD, Weiner LM. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res. 2001. 61:4750–4755.26. Rudnick SI, Lou J, Shaller CC, Tang Y, Klein-Szanto AJ, Weiner LM, Marks JD, Adams GP. Influence of affinity and antigen internalization on the uptake and penetration of Anti-HER2 antibodies in solid tumors. Cancer Res. 2011. 71:2250–2259.
Article27. Kamat V, Donaldson JM, Kari C, Quadros MR, Lelkes PI, Chaiken I, Cocklin S, Williams JC, Papazoglou E, Rodeck U. Enhanced EGFR inhibition and distinct epitope recognition by EGFR antagonistic mAbs C225 and 425. Cancer Biol Ther. 2008. 7:726–733.
Article28. Schmiedel J, Blaukat A, Li S, Knöchel T, Ferguson KM. Matuzumab binding to EGFR prevents the conformational rearrangement required for dimerization. Cancer Cell. 2008. 13:365–373.
Article29. Li S, Kussie P, Ferguson KM. Structural basis for EGF receptor inhibition by the therapeutic antibody IMC-11F8. Structure. 2008. 16:216–227.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Conversion of a murine monoclonal antibody A13 targeting epidermal growth factor receptor to a human monoclonal antibody by guided selection
- Expression of Epidermal Growth Factor, Transforming Growth Factor-alphaand Epidermal Growth Factor Receptor in Human Trophoblast and Decidua
- Psoriasis Induced by Trastuzumab (Herceptin(R))
- Prognostic significance of epidermal growth factor receptor expression in human gastric carcinoma
- Amplification of epidermal growth factor receptor gene in primary cervical cancer