Yonsei Med J.
2017 May;58(3):604-612. 10.3349/ymj.2017.58.3.604.
Value of Serum Cystatin C Measurement in the Diagnosis of Sepsis-Induced Kidney Injury and Prediction of Renal Function Recovery
- Affiliations
-
- 1Division of Pulmonology and Critical Care Medicine, Department of Internal Medicine, Institute of Chest Disease, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. augustin76md@yuhs.ac
- 2Division of Respiratory and Critical Care Medicine, Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
- KMID: 2419119
- DOI: http://doi.org/10.3349/ymj.2017.58.3.604
Abstract
- PURPOSE
Acute kidney injury (AKI) is common in critically ill patients. Serum cystatin C has emerged as a reliable marker of AKI. We sought to assess the value of serum cystatin C for early detection and prediction of renal function recovery in patients with sepsis.
MATERIALS AND METHODS
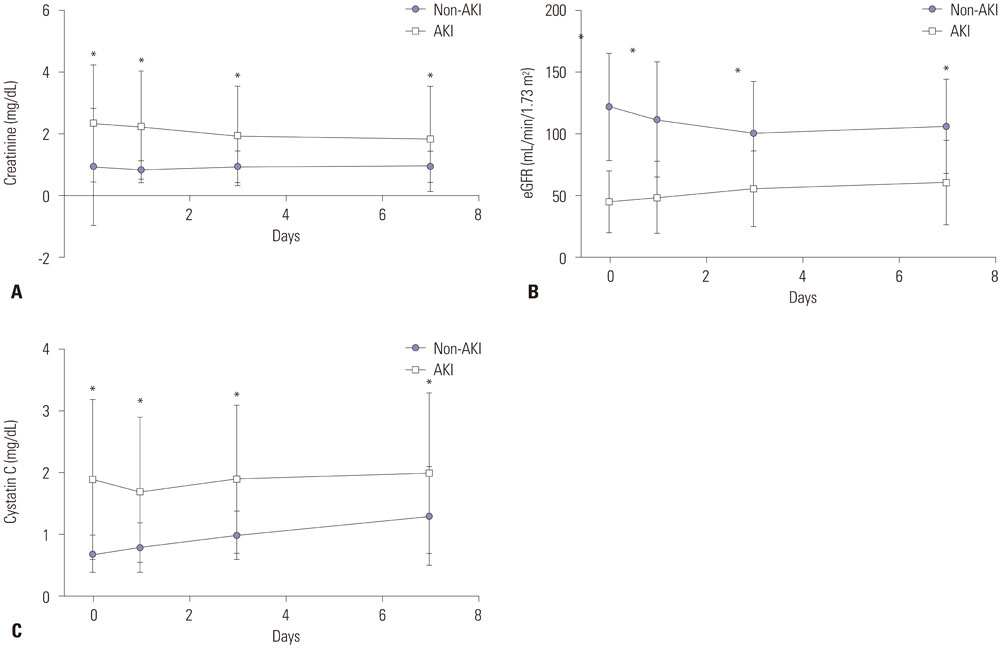
Sepsis patients (113 AKI patients and 49 non-AKI patients) admitted to the intensive care unit (ICU) were included. Serum creatinine and cystatin C levels and glomerular filtration rate were measured on days 0, 1, 3, and 7.
RESULTS
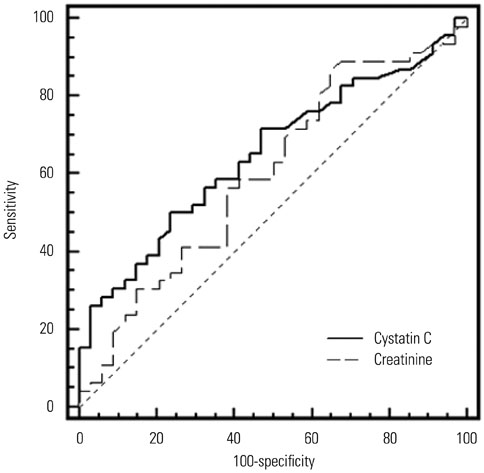
Serum cystatin C levels were significantly higher in AKI patients than in non-AKI patients at all time points. Multivariate analysis showed that only serum cystatin C levels on day 0 were associated with AKI development [odds ratio (OR)=19.30; 95% confidence interval (CI)= 2.58-144.50, p<0.001]. Linear mixed model analysis showed significant variation in cystatin C levels between the recovery and non-recovery groups over time (p=0.001). High levels of serum cystatin C at day 0 (OR=1.64; 95% CI=1.00-2.68, p=0.048) were associated with recovery of AKI.
CONCLUSION
Serum cystatin C level was found to be associated with the development and worsening of AKI in ICU patients with sepsis.
Keyword
MeSH Terms
-
Acute Kidney Injury/*blood/*diagnosis
Adult
Aged
Biomarkers/blood
Case-Control Studies
Creatinine/*blood
Critical Care
Critical Illness
Cystatin C/*blood
Disease Progression
Early Diagnosis
Female
Glomerular Filtration Rate
Humans
Intensive Care Units
Kidney Function Tests
Male
Middle Aged
Multivariate Analysis
Odds Ratio
Predictive Value of Tests
Prospective Studies
Recovery of Function
Sensitivity and Specificity
Sepsis/blood/complications/*diagnosis
Time Factors
Biomarkers
Cystatin C
Creatinine
Figure
Cited by 1 articles
-
Clinical Values of Combined Detection of Serum Cystatin C, β2-Microglobulin, and Urine Transferrin in Diagnosing Early Primary Glomerulonephritis
Xueqi Zhang, Xueying Bao, Cuicui Wu, Binxian Li, Mingcheng Li
Ann Lab Med. 2025;45(3):329-333. doi: 10.3343/alm.2024.0489.
Reference
-
1. Cole L, Bellomo R, Silvester W, Reeves JH. A prospective, multicenter study of the epidemiology, management, and outcome of severe acute renal failure in a “closed” ICU system. Am J Respir Crit Care Med. 2000; 162:191–196.
Article2. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005; 294:813–818.
Article3. Gursel G, Demir N. Incidence and risk factors for the development of acute renal failure in patients with ventilator-associated pneumonia. Nephrology (Carlton). 2006; 11:159–164.
Article4. Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006; 10:R73.5. Zhang Z. Biomarkers, diagnosis and management of sepsis-induced acute kidney injury: a narrative review. Heart Lung Vessel. 2015; 7:64–73.6. Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004; 351:159–169.
Article7. Nejat M, Pickering JW, Walker RJ, Endre ZH. Rapid detection of acute kidney injury by plasma cystatin C in the intensive care unit. Nephrol Dial Transplant. 2010; 25:3283–3289.
Article8. Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004; 66:1115–1122.
Article9. Bagshaw SM, Gibney RT. Conventional markers of kidney function. Crit Care Med. 2008; 36:S152–S158.
Article10. Aydoğdu M, Gürsel G, Sancak B, Yeni S, Sarı G, Tas¸yürek S, et al. The use of plasma and urine neutrophil gelatinase associated lipocalin (NGAL) and Cystatin C in early diagnosis of septic acute kidney injury in critically ill patients. Dis Markers. 2013; 34:237–246.
Article11. Bagshaw SM, Bellomo R. Cystatin C in acute kidney injury. Curr Opin Crit Care. 2010; 16:533–539.
Article12. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992; 20:864–874.13. Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008; 36:296–327.
Article14. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup. Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004; 8:R204–R212.15. Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med. 2016; 4:91.
Article16. Zhang Z. Model building strategy for logistic regression: purposeful selection. Ann Transl Med. 2016; 4:111.
Article17. Zhang Z. Residuals and regression diagnostics: focusing on logistic regression. Ann Transl Med. 2016; 4:195.
Article18. Abraham BP, Frazier EA, Morrow WR, Blaszak RT, Devarajan P, Mitsnefes M, et al. Cystatin C and neutrophil gelatinase-associated lipocalin as markers of renal function in pediatric heart transplant recipients. Pediatr Transplant. 2011; 15:564–569.
Article19. Doi K, Yuen PS, Eisner C, Hu X, Leelahavanichkul A, Schnermann J, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009; 20:1217–1221.
Article20. Mårtensson J, Martling CR, Oldner A, Bell M. Impact of sepsis on levels of plasma cystatin C in AKI and non-AKI patients. Nephrol Dial Transplant. 2012; 27:576–581.
Article21. Vinge E, Lindergård B, Nilsson-Ehle P, Grubb A. Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest. 1999; 59:587–592.
Article22. Segarra A, de la Torre J, Ramos N, Quiroz A, Garjau M, Torres I, et al. Assessing glomerular filtration rate in hospitalized patients: a comparison between CKD-EPI and four cystatin C-based equations. Clin J Am Soc Nephrol. 2011; 6:2411–2420.
Article23. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002; 40:221–226.
Article24. Cruz DN, de Geus HR, Bagshaw SM. Biomarker strategies to predict need for renal replacement therapy in acute kidney injury. Semin Dial. 2011; 24:124–131.
Article25. Ahlström A, Tallgren M, Peltonen S, Pettilä V. Evolution and predictive power of serum cystatin C in acute renal failure. Clin Nephrol. 2004; 62:344–350.
Article26. Zhang Z, Lu B, Sheng X, Jin N. Cystatin C in prediction of acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis. 2011; 58:356–365.
Article27. Di Nardo M, Ficarella A, Ricci Z, Luciano R, Stoppa F, Picardo S, et al. Impact of severe sepsis on serum and urinary biomarkers of acute kidney injury in critically ill children: an observational study. Blood Purif. 2013; 35:172–176.
Article28. Dai X, Zeng Z, Fu C, Zhang S, Cai Y, Chen Z. Diagnostic value of neutrophil gelatinase-associated lipocalin, cystatin C, and soluble triggering receptor expressed on myeloid cells-1 in critically ill patients with sepsis-associated acute kidney injury. Crit Care. 2015; 19:223.
Article29. Roos JF, Doust J, Tett SE, Kirkpatrick CM. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children--a meta-analysis. Clin Biochem. 2007; 40:383–391.
Article30. Perianayagam MC, Seabra VF, Tighiouart H, Liangos O, Jaber BL. Serum cystatin C for prediction of dialysis requirement or death in acute kidney injury: a comparative study. Am J Kidney Dis. 2009; 54:1025–1033.
Article31. Royakkers AA, Korevaar JC, van Suijlen JD, Hofstra LS, Kuiper MA, Spronk PE, et al. Serum and urine cystatin C are poor biomarkers for acute kidney injury and renal replacement therapy. Intensive Care Med. 2011; 37:493–501.
Article32. Bökenkamp A, Laarman CA, Braam KI, van Wijk JA, Kors WA, Kool M, et al. Effect of corticosteroid therapy on low-molecular weight protein markers of kidney function. Clin Chem. 2007; 53:2219–2221.33. Fricker M, Wiesli P, Brändle M, Schwegler B, Schmid C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003; 63:1944–1947.
Article34. Yokoyama H, Inoue T, Node K. Effect of insulin-unstimulated diabetic therapy with miglitol on serum cystatin C level and its clinical significance. Diabetes Res Clin Pract. 2009; 83:77–82.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cystatin C as a Renal Function Marker for Neonates
- Clinical Usefulness of Serum Cystatin C as a Marker of Renal Function
- Current knowledge about biomarkers of acute kidney injury in liver cirrhosis
- Serum Cystatin C for the Evaluation of Renal Function in the Spinal Cord Injured Patients
- New Biomarkers of Acute Kidney Injury and the Cardio-renal Syndrome