Int J Stem Cells.
2018 Jun;11(1):61-67. 10.15283/ijsc17054.
Improvement of Cell Cycle Lifespan and Genetic Damage Susceptibility of Human Mesenchymal Stem Cells by Hypoxic Priming
- Affiliations
-
- 1Department of Molecular Cell Biology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Suwon, Korea. cwlee1234@skku.edu
- 2Department of Health Sciences and Technology, SAIHST, Sungkyunkwan University, Suwon, Korea.
- 3Division of Vascular Surgery, Samsung Medical Center, Sungkyunwan University School of Medicine, Seoul, Korea. dikim@skku.edu
- KMID: 2413501
- DOI: http://doi.org/10.15283/ijsc17054
Abstract
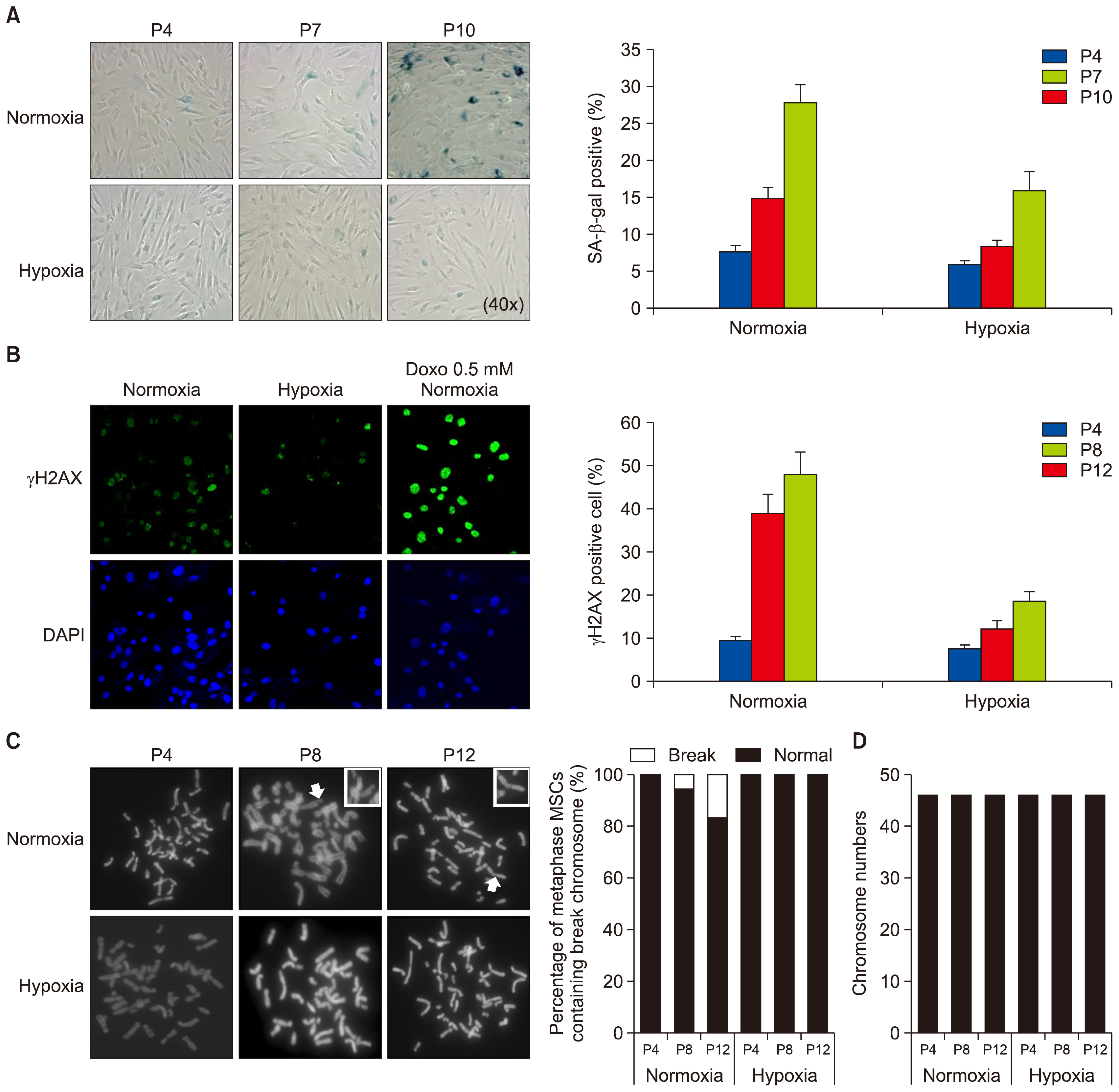
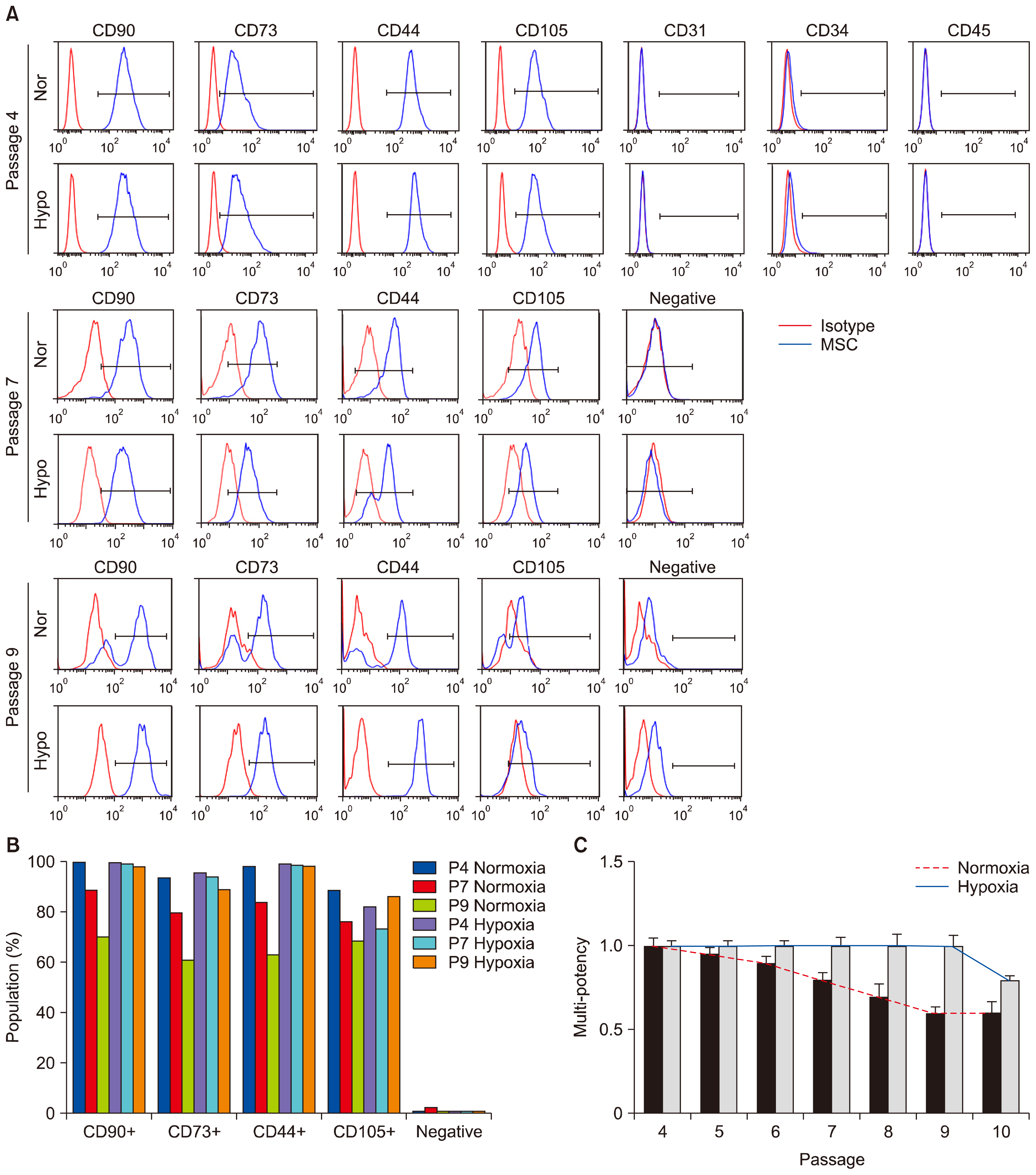
- Hypoxic culture is widely recognized as a method to efficiently expand human mesenchymal stem cells (MSCs) without loss of stem cell properties. However, the molecular basis of how hypoxia priming benefits MSC expansion remains unclear. We report that hypoxic priming markedly extends the cell cycle lifespan rather than augmenting the multipotency of MSC differentiation lineage. Hypoxic priming does not affect to chromosome damage but significantly attenuates the susceptibility of chromosome damage. Our results provide important evidence that multipotency of human MSCs by hypoxic priming is determined by cell cycle lifespan.
Figure
Cited by 1 articles
-
Monitoring Glutathione Dynamics and Heterogeneity in Living Stem Cells
Eui Man Jeong, Ji-Woong Shin, Jisun Lim, Ju Hwan Kim, Hyewon Kang, Yingfu Yin, Hye-Mi Kim, YongHwan Kim, Sun-Gi Kim, Heun-Soo Kang, Dong-Myung Shin, Kihang Choi, In-Gyu Kim
Int J Stem Cells. 2019;12(2):367-379. doi: 10.15283/ijsc18151.
Reference
-
References
1. Anderson DJ, Gage FH, Weissman IL. Can stem cells cross lineage boundaries? Nat Med. 2001; 7:393–395. DOI: 10.1038/86439. PMID: 11283651.
Article2. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002; 418:41–49. DOI: 10.1038/nature00870. PMID: 12077603.
Article3. Mohammadian M, Shamsasenjan K, Lotfi Nezhad P, Talebi M, Jahedi M, Nickkhah H, Minayi N, Movassagh Pour A. Mesenchymal stem cells: new aspect in cell-based regenerative therapy. Adv Pharm Bull. 2013; 3:433–437. PMID: 24312873. PMCID: 3848236.4. Momin EN, Mohyeldin A, Zaidi HA, Vela G, Quiñones-Hinojosa A. Mesenchymal stem cells: new approaches for the treatment of neurological diseases. Curr Stem Cell Res Ther. 2010; 5:326–344. DOI: 10.2174/157488810793351631. PMID: 20528757.
Article5. da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006; 119:2204–2213. DOI: 10.1242/jcs.02932. PMID: 16684817.
Article6. Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003; 21:105–110. DOI: 10.1634/stemcells.21-1-105. PMID: 12529557.
Article7. Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004; 22:649–658. DOI: 10.1634/stemcells.22-5-649. PMID: 15342929.
Article8. Davy P, Allsopp R. Hypoxia: are stem cells in it for the long run? Cell Cycle. 2011; 10:206–211. DOI: 10.4161/cc.10.2.14535. PMID: 21239881.
Article9. Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, Hung SC. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011; 117:459–469. DOI: 10.1182/blood-2010-05-287508.
Article10. Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008; 26:2173–2182. DOI: 10.1634/stemcells.2007-1104. PMID: 18511601. PMCID: 3017477.
Article11. Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011; 9:298–310. DOI: 10.1016/j.stem.2011.09.010. PMID: 21982230.
Article12. Mathieu J, Zhou W, Xing Y, Sperber H, Ferreccio A, Agoston Z, Kuppusamy KT, Moon RT, Ruohola-Baker H. Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell. 2014; 14:592–605. DOI: 10.1016/j.stem.2014.02.012. PMID: 24656769. PMCID: 4028142.
Article13. Zhang J, Nuebel E, Daley GQ, Koehler CM, Teitell MA. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell. 2012; 11:589–595. DOI: 10.1016/j.stem.2012.10.005. PMID: 23122286. PMCID: 3492890.
Article14. Theus MH, Wei L, Cui L, Francis K, Hu X, Keogh C, Yu SP. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008; 210:656–670. DOI: 10.1016/j.expneurol.2007.12.020. PMID: 18279854.
Article15. Francis KR, Wei L. Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis. 2010; 1:e22. DOI: 10.1038/cddis.2009.22.
Article16. Jaussaud J, Biais M, Calderon J, Chevaleyre J, Duchez P, Ivanovic Z, Couffinhal T, Barandon L. Hypoxia-preconditioned mesenchymal stromal cells improve cardiac function in a swine model of chronic myocardial ischaemia. Eur J Cardiothorac Surg. 2013; 43:1050–1057. DOI: 10.1093/ejcts/ezs549.
Article17. Muscari C, Giordano E, Bonafè F, Govoni M, Pasini A, Guarnieri C. Priming adult stem cells by hypoxic pretreatments for applications in regenerative medicine. J Biomed Sci. 2013; 20:63. DOI: 10.1186/1423-0127-20-63. PMID: 23985033. PMCID: 3765890.
Article18. Chang CP, Chio CC, Cheong CU, Chao CM, Cheng BC, Lin MT. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin Sci (Lond). 2013; 124:165–176. DOI: 10.1042/CS20120226.
Article19. Mottaghi S, Larijani B, Sharifi AM. Apelin 13: a novel approach to enhance efficacy of hypoxic preconditioned mesenchymal stem cells for cell therapy of diabetes. Med Hypotheses. 2012; 79:717–718. DOI: 10.1016/j.mehy.2012.08.007. PMID: 22981008.
Article20. Watanabe S, Arimura Y, Nagaishi K, Isshiki H, Onodera K, Nasuno M, Yamashita K, Idogawa M, Naishiro Y, Murata M, Adachi Y, Fujimiya M, Imai K, Shinomura Y. Conditioned mesenchymal stem cells produce pleiotropic gut trophic factors. J Gastroenterol. 2014; 49:270–282. DOI: 10.1007/s00535-013-0901-3.
Article21. Liu H, Liu S, Li Y, Wang X, Xue W, Ge G, Luo X. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS One. 2012; 7:e34608. DOI: 10.1371/journal.pone.0034608. PMID: 22511954. PMCID: 3325280.
Article22. Yu X, Lu C, Liu H, Rao S, Cai J, Liu S, Kriegel AJ, Greene AS, Liang M, Ding X. Hypoxic preconditioning with cobalt of bone marrow mesenchymal stem cells improves cell migration and enhances therapy for treatment of ischemic acute kidney injury. PLoS One. 2013; 8:e62703. DOI: 10.1371/journal.pone.0062703. PMID: 23671625. PMCID: 3650042.
Article23. Yu J, Yin S, Zhang W, Gao F, Liu Y, Chen Z, Zhang M, He J, Zheng S. Hypoxia preconditioned bone marrow mesenchymal stem cells promote liver regeneration in a rat massive hepatectomy model. Stem Cell Res Ther. 2013; 4:83. DOI: 10.1186/scrt234. PMID: 23856418. PMCID: 3854783.
Article24. Bonfield TL, Caplan AI. Adult mesenchymal stem cells: an innovative therapeutic for lung diseases. Discov Med. 2010; 9:337–345. PMID: 20423678.25. Inamdar AC, Inamdar AA. Mesenchymal stem cell therapy in lung disorders: pathogenesis of lung diseases and mechanism of action of mesenchymal stem cell. Exp Lung Res. 2013; 39:315–327. DOI: 10.3109/01902148.2013.816803. PMID: 23992090.
Article26. Chacko SM, Ahmed S, Selvendiran K, Kuppusamy ML, Khan M, Kuppusamy P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol. 2010; 299:C1562–C1570. DOI: 10.1152/ajpcell.00221.2010. PMID: 20861473. PMCID: 3006322.
Article27. Ohnishi S, Yasuda T, Kitamura S, Nagaya N. Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells. Stem Cells. 2007; 25:1166–1177. DOI: 10.1634/stemcells.2006-0347. PMID: 17289933.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Enhanced Anti-Cancer Effects of Conditioned Medium from Hypoxic Human Umbilical Cord–Derived Mesenchymal Stem Cells
- Recent Trends and Strategies in Stem Cell Therapy for Alzheimer's Disease
- FGF-17 from Hypoxic Human Wharton's Jelly-Derived Mesenchymal Stem Cells Is Responsible for Maintenance of Cell Proliferation at Late Passages
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Cell cycle checkpoint control