Int J Stem Cells.
2019 Jul;12(2):291-303. 10.15283/ijsc19002.
Enhanced Anti-Cancer Effects of Conditioned Medium from Hypoxic Human Umbilical Cord–Derived Mesenchymal Stem Cells
- Affiliations
-
- 1Division of Vascular Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. dikim@skku.edu
- 2Sungkyunkwan University School of Chemical Engineering, Suwon, Korea.
- KMID: 2465899
- DOI: http://doi.org/10.15283/ijsc19002
Abstract
- BACKGROUND AND OBJECTIVES
There have been contradictory reports on the pro-cancer or anti-cancer effects of mesenchymal stem cells. In this study, we investigated whether conditioned medium (CM) from hypoxic human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) (H-CM) showed enhanced anti-cancer effects compared with CM from normoxic hUC-MSCs (N-CM).
METHODS AND RESULTS
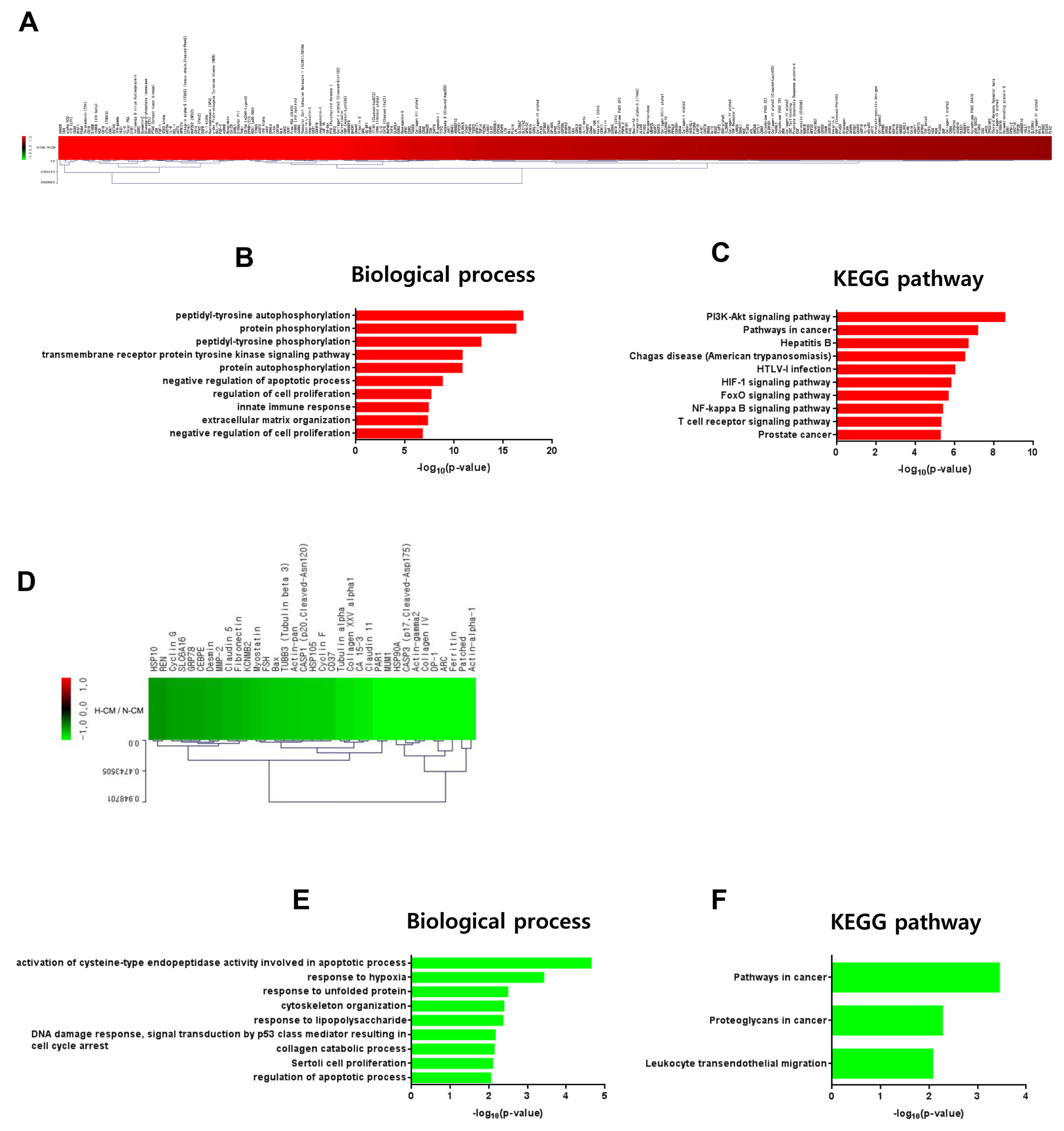
Compared with N-CM, H-CM not only strongly reduced cell viability and increased apoptosis of human cervical cancer cells (HeLa cells), but also increased caspase-3/7 activity, decreased mitochondrial membrane potential (MMP), and induced cell cycle arrest. In contrast, cell viability, apoptosis, MMP, and cell cycle of human dermal fibroblast (hDFs) were not significantly changed by either CM whereas caspase-3/7 activity was decreased by H-CM. Protein antibody array showed that activin A, Beta IG-H3, TIMP-2, RET, and IGFBP-3 were upregulated in H-CM compared with N-CM. Intracellular proteins that were upregulated by H-CM in HeLa cells were represented by apoptosis and cell cycle arrest terms of biological processes of Gene Ontology (GO), and by cell cycle of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. In hDFs, negative regulation of apoptosis in biological process of GO and PI3K-Akt signaling pathway of KEGG pathways were represented.
CONCLUSIONS
H-CM showed enhanced anti-cancer effects on HeLa cells but did not influence cell viability or apoptosis of hDFs and these different effects were supported by profiling of secretory proteins in both kinds of CM and intracellular signaling of HeLa cells and hDFs.
MeSH Terms
-
Activins
Anoxia
Apoptosis
Biological Processes
Cell Cycle
Cell Cycle Checkpoints
Cell Survival
Culture Media, Conditioned*
Fibroblasts
Gene Ontology
Genome
HeLa Cells
Humans*
Insulin-Like Growth Factor Binding Protein 3
Membrane Potential, Mitochondrial
Mesenchymal Stromal Cells*
Tissue Inhibitor of Metalloproteinase-2
Uterine Cervical Neoplasms
Activins
Culture Media, Conditioned
Insulin-Like Growth Factor Binding Protein 3
Tissue Inhibitor of Metalloproteinase-2
Figure
Reference
-
References
1. Rhee KJ, Lee JI, Eom YW. Mesenchymal stem cell-mediated effects of tumor support or suppression. Int J Mol Sci. 2015; 16:30015–30033. DOI: 10.3390/ijms161226215. PMID: 26694366. PMCID: PMC4691158.
Article2. Saito K, Sakaguchi M, Maruyama S, Iioka H, Putranto EW, Sumardika IW, Tomonobu N, Kawasaki T, Homma K, Kondo E. Stromal mesenchymal stem cells facilitate pancreatic cancer progression by regulating specific secretory molecules through mutual cellular interaction. J Cancer. 2018; 9:2916–2929. DOI: 10.7150/jca.24415. PMID: 30123360. PMCID: PMC6096376.
Article3. Gonzalez ME, Martin EE, Anwar T, Arellano-Garcia C, Medhora N, Lama A, Chen YC, Tanager KS, Yoon E, Kidwell KM, Ge C, Franceschi RT, Kleer CG. Mesenchymal stem cell-induced DDR2 mediates stromal-breast cancer interactions and metastasis growth. Cell Rep. 2017; 18:1215–1228. DOI: 10.1016/j.celrep.2016.12.079. PMID: 28147276. PMCID: PMC5332146.
Article4. Bu S, Wang Q, Zhang Q, Sun J, He B, Xiang C, Liu Z, Lai D. Human endometrial mesenchymal stem cells exhibit intrinsic anti-tumor properties on human epithelial ovarian cancer cells. Sci Rep. 2016; 6:37019. DOI: 10.1038/srep37019. PMID: 27845405. PMCID: PMC5109482.
Article5. Reza AM, Choi YJ, Yasuda H, Kim JH. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci Rep. 2016; 6:38498. DOI: 10.1038/srep38498. PMID: 27929108. PMCID: PMC5143979.
Article6. Pacioni S, D’Alessandris QG, Giannetti S, Morgante L, Coccè V, Bonomi A, Buccarelli M, Pascucci L, Alessandri G, Pessina A, Ricci-Vitiani L, Falchetti ML, Pallini R. Human mesenchymal stromal cells inhibit tumor growth in orthotopic glioblastoma xenografts. Stem Cell Res Ther. 2017; 8:53. DOI: 10.1186/s13287-017-0516-3. PMID: 28279193. PMCID: PMC5345323.
Article7. de Melo SM, Bittencourt S, Ferrazoli EG, da Silva CS, da Cunha FF, da Silva FH, Stilhano RS, Denapoli PM, Zanetti BF, Martin PK, Silva LM, dos Santos AA, Baptista LS, Longo BM, Han SW. The anti-tumor effects of adipose tissue mesenchymal stem cell transduced with HSV-Tk gene on U-87-driven brain tumor. PLoS One. 2015; 10:e0128–922. DOI: 10.1371/journal.pone.0128922. PMID: 26067671. PMCID: PMC4467037.
Article8. Yao S, Li X, Liu J, Sun Y, Wang Z, Jiang Y. Maximized nanodrug-loaded mesenchymal stem cells by a dual drug-loaded mode for the systemic treatment of metastatic lung cancer. Drug Deliv. 2017; 24:1372–1383. DOI: 10.1080/10717544.2017.1375580. PMID: 28920712.
Article9. Li L, Jaiswal PK, Makhoul G, Jurakhan R, Selvasandran K, Ridwan K, Cecere R. Hypoxia modulates cell migration and proliferation in placenta-derived mesenchymal stem cells. J Thorac Cardiovasc Surg. 2017; 154:543–552.e3. DOI: 10.1016/j.jtcvs.2017.03.141. PMID: 28526501.
Article10. Oh JS, Ha Y, An SS, Khan M, Pennant WA, Kim HJ, Yoon DH, Lee M, Kim KN. Hypoxia-preconditioned adipose tissue-derived mesenchymal stem cell increase the survival and gene expression of engineered neural stem cells in a spinal cord injury model. Neurosci Lett. 2010; 472:215–219. DOI: 10.1016/j.neulet.2010.02.008. PMID: 20153400.
Article11. Drela K, Sarnowska A, Siedlecka P, Szablowska-Gadomska I, Wielgos M, Jurga M, Lukomska B, Domanska-Janik K. Low oxygen atmosphere facilitates proliferation and maintains undifferentiated state of umbilical cord mesenchymal stem cells in an hypoxia inducible factor-dependent manner. Cytotherapy. 2014; 16:881–892. DOI: 10.1016/j.jcyt.2014.02.009. PMID: 24726658.
Article12. Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, Hung SC. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011; 117:459–469. DOI: 10.1182/blood-2010-05-287508. PMID: 20952688.
Article13. Fujisawa K, Takami T, Okada S, Hara K, Matsumoto T, Yamamoto N, Yamasaki T, Sakaida I. Analysis of metabolomic changes in mesenchymal stem cells on treatment with desferrioxamine as a hypoxia mimetic compared with hypoxic conditions. Stem Cells. 2018; 36:1226–1236. DOI: 10.1002/stem.2826. PMID: 29577517.
Article14. Han KH, Kim AK, Kim MH, Kim DH, Go HN, Kang D, Chang JW, Choi SW, Kang KS, Kim DI. Protein profiling and angiogenic effect of hypoxia-cultured human umbilical cord blood-derived mesenchymal stem cells in hindlimb ischemia. Tissue Cell. 2017; 49:680–690. DOI: 10.1016/j.tice.2017.09.006. PMID: 28958480.
Article15. Song SW, Kim KE, Choi JW, Lee CY, Lee J, Seo HH, Lim KH, Lim S, Lee S, Kim SW, Hwang KC. Proteomic analysis and identification of paracrine factors in mesenchymal stem cell-conditioned media under hypoxia. Cell Physiol Biochem. 2016; 40:400–410. DOI: 10.1159/000452555. PMID: 27866198.
Article16. Chen L, Xu Y, Zhao J, Zhang Z, Yang R, Xie J, Liu X, Qi S. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One. 2014; 9:e96161. DOI: 10.1371/journal.pone.0096161. PMID: 24781370. PMCID: PMC4004560.
Article17. Han KH, Kim AK, Kim MH, Kim DH, Go HN, Kim DI. Enhancement of angiogenic effects by hypoxia-preconditioned human umbilical cord-derived mesenchymal stem cells in a mouse model of hindlimb ischemia. Cell Biol Int. 2016; 40:27–35. DOI: 10.1002/cbin.10519. PMID: 26222206.
Article18. Lan YW, Choo KB, Chen CM, Hung TH, Chen YB, Hsieh CH, Kuo HP, Chong KY. Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther. 2015; 6:97. DOI: 10.1186/s13287-015-0081-6. PMID: 25986930. PMCID: PMC4487587.
Article19. Liu YY, Chiang CH, Hung SC, Chian CF, Tsai CL, Chen WC, Zhang H. Hypoxia-preconditioned mesenchymal stem cells ameliorate ischemia/reperfusion-induced lung injury. PLoS One. 2017; 12:e0187637. DOI: 10.1371/journal.pone.0187637. PMID: 29117205. PMCID: PMC5678873.
Article20. Shin HS, Lee S, Kim YM, Lim JY. Hypoxia-activated adipose mesenchymal stem cells prevents irradiation-induced salivary hypofunction by enhanced paracrine effect through fibroblast growth factor 10. Stem Cells. 2018; 36:1020–1032. DOI: 10.1002/stem.2818. PMID: 29569790.
Article21. Hung SP, Yang MH, Tseng KF, Lee OK. Hypoxia-induced secretion of TGF-β1 in mesenchymal stem cell promotes breast cancer cell progression. Cell Transplant. 2013; 22:1869–1882. DOI: 10.3727/096368912X657954. PMID: 23067574.
Article22. Katik I, Mackenzie-Kludas C, Nicholls C, Jiang FX, Zhou S, Li H, Liu JP. Activin inhibits telomerase activity in cancer. Biochem Biophys Res Commun. 2009; 389:668–672. DOI: 10.1016/j.bbrc.2009.09.055. PMID: 19769941.
Article23. Bashir M, Damineni S, Mukherjee G, Kondaiah P. Activin-A signaling promotes epithelial-mesenchymal transition, invasion, and metastatic growth of breast cancer. NPJ Breast Cancer. 2015; 1:15007. DOI: 10.1038/npjbcancer.2015.7. PMID: 28721365. PMCID: PMC5515205.
Article24. Hu J, Wang X, Wei SM, Tang YH, Zhou Q, Huang CX. Activin A stimulates the proliferation and differentiation of cardiac fibroblasts via the ERK1/2 and p38-MAPK pathways. Eur J Pharmacol. 2016; 789:319–327. DOI: 10.1016/j.ejphar.2016.07.053. PMID: 27477354.
Article25. Ween MP, Oehler MK, Ricciardelli C. Transforming growth Factor-Beta-Induced Protein (TGFBI)/(βig-H3): a matrix protein with dual functions in ovarian cancer. Int J Mol Sci. 2012; 13:10461–10477. DOI: 10.3390/ijms130810461. PMID: 22949874. PMCID: PMC3431872.
Article26. LeBaron RG, Bezverkov KI, Zimber MP, Pavelec R, Skonier J, Purchio AF. Beta IG-H3, a novel secretory protein inducible by transforming growth factor-beta, is present in normal skin and promotes the adhesion and spreading of dermal fibroblasts in vitro. J Invest Dermatol. 1995; 104:844–849. DOI: 10.1111/1523-1747.ep12607024. PMID: 7738366.
Article27. Valacca C, Tassone E, Mignatti P. TIMP-2 interaction with MT1-MMP activates the AKT pathway and protects tumor cells from apoptosis. PLoS One. 2015; 10:e0136797. DOI: 10.1371/journal.pone.0136797. PMID: 26331622. PMCID: PMC4558019.
Article28. Chen Z, Zhu J, Zhu Y, Wang J. MicroRNA-616 promotes the progression of ovarian cancer by targeting TIMP2. Oncol Rep. 2018; 39:2960–2968. DOI: 10.3892/or.2018.6368. PMID: 29658596.
Article29. Dohi T, Miyake K, Aoki M, Ogawa R, Akaishi S, Shimada T, Okada T, Hyakusoku H. Tissue inhibitor of metalloproteinase-2 suppresses collagen synthesis in cultured keloid fibroblasts. Plast Reconstr Surg Glob Open. 2015; 3:e520. DOI: 10.1097/GOX.0000000000000503. PMID: 26495233. PMCID: PMC4596445.
Article30. Luo Y, Tsuchiya KD, Il Park D, Fausel R, Kanngurn S, Welcsh P, Dzieciatkowski S, Wang J, Grady WM. RET is a potential tumor suppressor gene in colorectal cancer. Oncogene. 2013; 32:2037–2047. DOI: 10.1038/onc.2012.225. PMID: 22751117. PMCID: PMC3465636.
Article31. Kodama Y, Asai N, Kawai K, Jijiwa M, Murakumo Y, Ichihara M, Takahashi M. The RET proto-oncogene: a molecular therapeutic target in thyroid cancer. Cancer Sci. 2005; 96:143–148. DOI: 10.1111/j.1349-7006.2005.00023.x. PMID: 15771616.
Article32. Naspi A, Panasiti V, Abbate F, Roberti V, Devirgiliis V, Curzio M, Borghi M, Lozupone F, Carotti S, Morini S, Gaudio E, Calvieri S, Londei P. Insulin-like-growth-factor-binding-protein-3 (IGFBP-3) contrasts melanoma progression in vitro and in vivo. PLoS One. 2014; 9:e98641. DOI: 10.1371/journal.pone.0098641. PMID: 24905466. PMCID: PMC4048209.
Article33. Izumi K, Kurosaka D, Iwata T, Oguchi Y, Tanaka Y, Mashima Y, Tsubota K. Involvement of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in corneal fibroblasts during corneal wound healing. Invest Ophthalmol Vis Sci. 2006; 47:591–598. DOI: 10.1167/iovs.05-0097. PMID: 16431955.
Article34. Schmid M, Simpson D, Gietl C. Programmed cell death in castor bean endosperm is associated with the accumulation and release of a cysteine endopeptidase from ricinosomes. Proc Natl Acad Sci U S A. 1999; 96:14159–14164. DOI: 10.1073/pnas.96.24.14159. PMID: 10570215. PMCID: PMC24207.
Article35. Yogosawa S, Yoshida K. Tumor suppressive role for kinases phosphorylating p53 in DNA damage-induced apoptosis. Cancer Sci. 2018; 109:3376–3382. DOI: 10.1111/cas.13792. PMID: 30191640. PMCID: PMC6215896.
Article36. Engeland K. Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM. Cell Death Differ. 2018; 25:114–132. DOI: 10.1038/cdd.2017.172.
Article37. Chun Yang X, Hui Zhao D, Bond Lau W, Qiang Liu K, Yu Tian J, Chao Cheng Z, Liang Ma X, Hua Liu J, Fan Q. lncRNA ENSMUST00000134285 increases MAPK11 activity, regulating aging-related myocardial apoptosis. J Gerontol A Biol Sci Med Sci. 2018; 73:1010–1017. DOI: 10.1093/gerona/gly020. PMID: 29415197.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of a Hair Tonic Containing Human Umbilical Cord Blood Mesenchymal Stem Cell-derived Conditioned Media in Patients with Androgenetic Alopecia
- Effect of Conditioned Medium from Human Umbilical Cord-Derived Mesenchymal Stromal Cells on Rejuvenation of Nucleus Pulposus Derived Stem/Progenitor Cells from Degenerated Intervertebral Disc
- FGF-17 from Hypoxic Human Wharton's Jelly-Derived Mesenchymal Stem Cells Is Responsible for Maintenance of Cell Proliferation at Late Passages
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Quantitative Tracking Tumor Suppression Efficiency of Human Umbilical Cord-Derived Mesenchymal Stem Cells by Bioluminescence Imaging in Mice Hepatoma Model