Int J Stem Cells.
2019 Jul;12(2):279-290. 10.15283/ijsc18042.
FGF-17 from Hypoxic Human Wharton's Jelly-Derived Mesenchymal Stem Cells Is Responsible for Maintenance of Cell Proliferation at Late Passages
- Affiliations
-
- 1Division of Vascular Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. dikim@skku.edu
- 2Stem Cell & Regenerative Medicine Institute, Research Institute for Future Medicine, Samsung Medical Center, Seoul, Korea.
- KMID: 2465898
- DOI: http://doi.org/10.15283/ijsc18042
Abstract
- BACKGROUND AND OBJECTIVES
Although it is well known that hypoxic culture conditions enhance proliferation of human mesenchymal stem cells, the exact mechanism is not fully understood. In this study, we investigated the effect of fibroblast growth factor (FGF)-17 from hypoxic human Wharton's Jelly-derived mesenchymal stem cells (hWJ-MSCs) on cell proliferation at late passages.
METHODS AND RESULTS
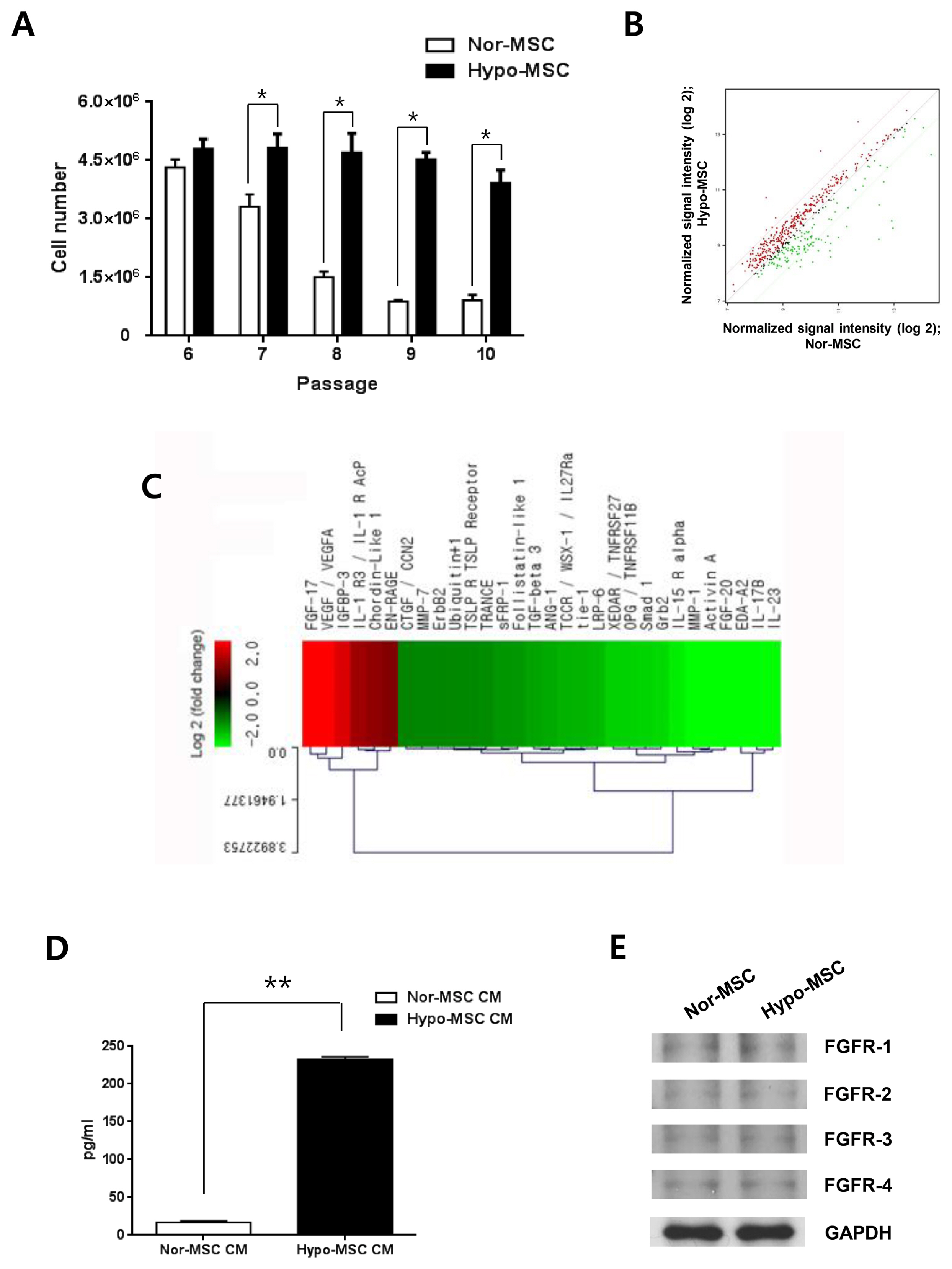
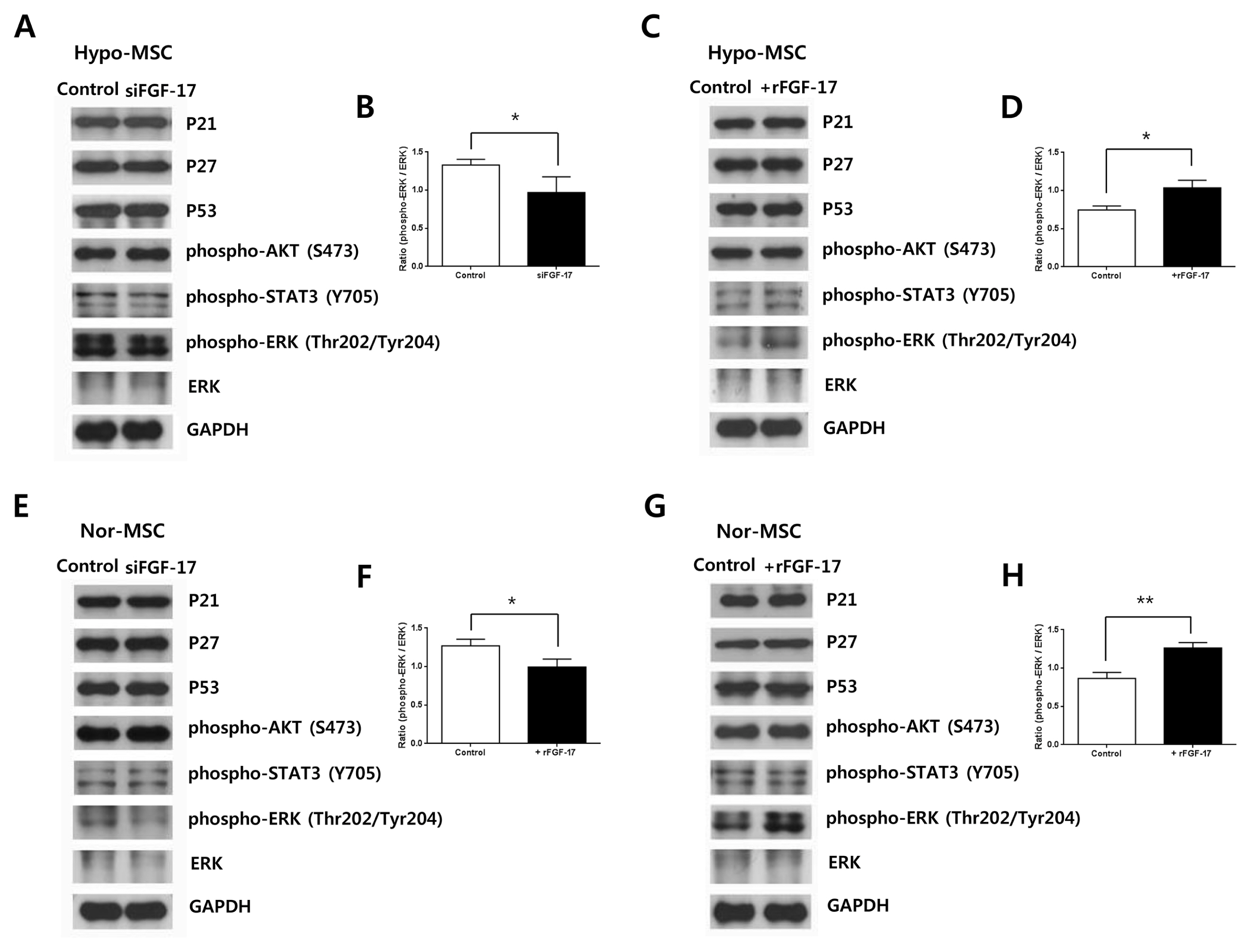
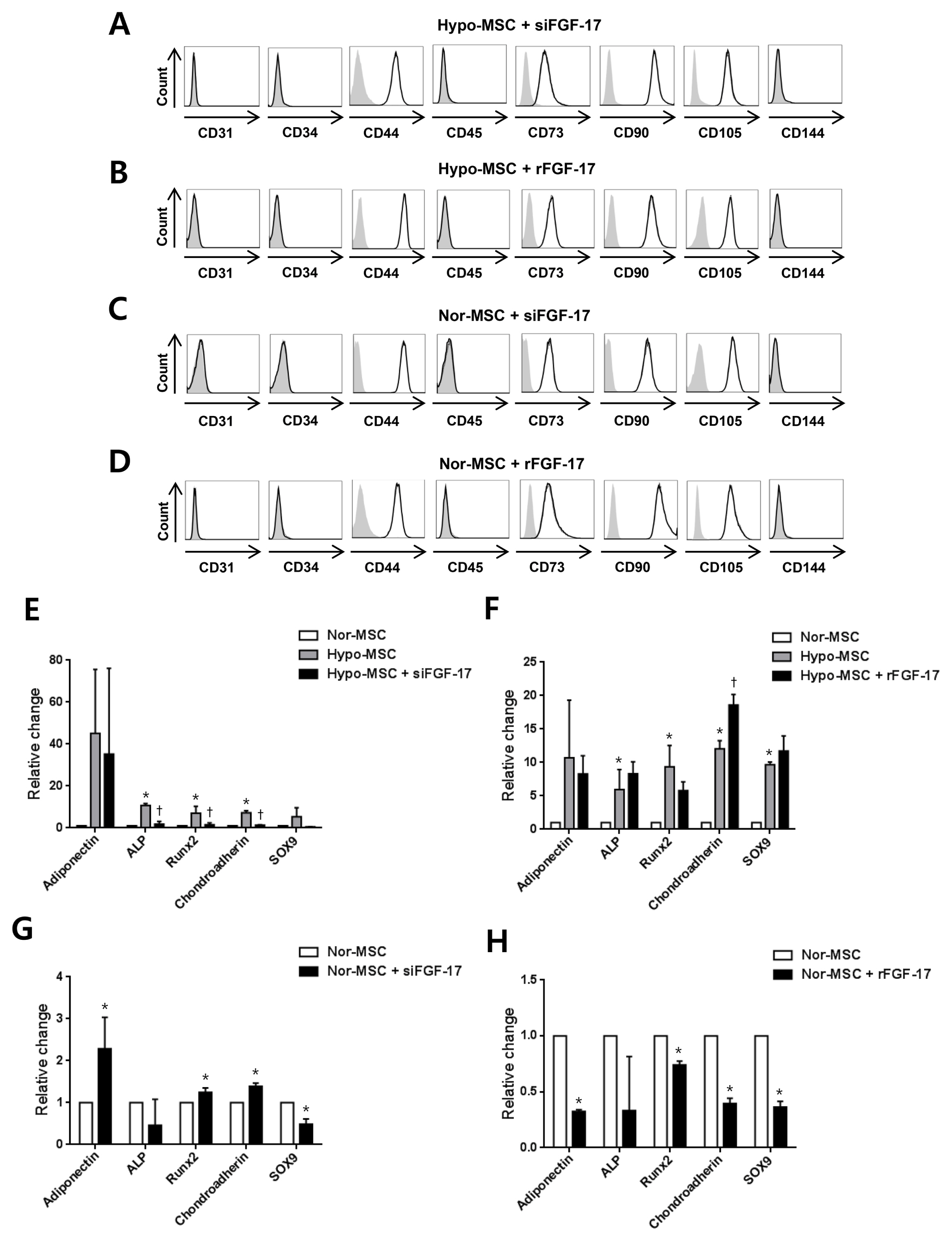
hWJ-MSCs were cultured in α-MEM medium supplemented with 10% fetal bovine serum (FBS) in normoxic (21% O₂) and hypoxic (1% O₂) conditions. Protein antibody array was performed to analyze secretory proteins in conditioned medium from normoxic and hypoxic hWJ-MSCs at passage 10. Cell proliferation of hypoxic hWJ-MSCs was increased compared with normoxic hWJ-MSCs from passage 7 to 10, and expression of secretory FGF-17 was highly increased in conditioned medium from hypoxic hWJ-MSCs at passage 10. Knockdown of FGF-17 in hypoxic and normoxic hWJ-MSCs decreased cell proliferation, whereas treatment of hypoxic and normoxic hWJ-MSCs with recombinant protein FGF-17 increased their proliferation. Signal transduction of FGF-17 in hypoxic and normoxic hWJ-MSCs involved the ERK1/2 pathway. Cell phenotypes were not changed under either condition. Differentiation-related genes adiponectin, Runx2, and chondroadherin were downregulated in normoxic hWJ-MSCs treated with rFGF-17, and upregulated by siFGF-17. Expression of alkaline phosphatase (ALP), Runx2, and chondroadherin was upregulated in hypoxic hWJ-MSCs, and this effect was rescued by transfection with siFGF-17. Only chondroadherin was upregulated in hypoxic hWJ-MSCs with rFGF-17.
CONCLUSIONS
In hypoxic culture conditions, FGF-17 from hypoxic hWJ-MSCs contributes to the maintenance of high proliferation at late passages through the ERK1/2 pathway.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Liu Y, Yan W, Tohme S, Chen M, Fu Y, Tian D, Lotze M, Tang D, Tsung A. Hypoxia induced HMGB1 and mitochondrial DNA interactions mediate tumor growth in hepatocellular carcinoma through Toll-like receptor 9. J Hepatol. 2015; 63:114–121. DOI: 10.1016/j.jhep.2015.02.009. PMID: 25681553. PMCID: PMC4475488.
Article2. Panigrahi GK, Praharaj PP, Peak TC, Long J, Singh R, Rhim JS, Abd Elmageed ZY, Deep G. Hypoxia-induced exosome secretion promotes survival of African-American and Caucasian prostate cancer cells. Sci Rep. 2018; 8:3853. DOI: 10.1038/s41598-018-22068-4. PMID: 29497081. PMCID: PMC5832762.
Article3. Porter KM, Kang BY, Adesina SE, Murphy TC, Hart CM, Sutliff RL. Chronic hypoxia promotes pulmonary artery endothelial cell proliferation through H2O2-induced 5-lipoxygenase. PLoS One. 2014; 9:e98532. DOI: 10.1371/journal.pone.0098532. PMID: 24906007. PMCID: PMC4048210.
Article4. Chai X, Sun D, Han Q, Yi L, Wu Y, Liu X. Hypoxia induces pulmonary arterial fibroblast proliferation, migration, differentiation and vascular remodeling via the PI3K/Akt/p70S6K signaling pathway. Int J Mol Med. 2018; 41:2461–2472. PMID: 29436587. PMCID: PMC5846667.
Article5. Chen R, Liu Y, Su Q, Yang Y, Wang L, Ma S, Yan J, Xue F, Wang J. Hypoxia stimulates proliferation of rat neural stem/progenitor cells by regulating mir-21: an in vitro study. Neurosci Lett. 2017; 661:71–76. DOI: 10.1016/j.neulet.2017.09.037. PMID: 28939387.
Article6. Nekanti U, Dastidar S, Venugopal P, Totey S, Ta M. Increased proliferation and analysis of differential gene expression in human Wharton’s jelly-derived mesenchymal stromal cells under hypoxia. Int J Biol Sci. 2010; 6:499–512. DOI: 10.7150/ijbs.6.499. PMID: 20877435. PMCID: PMC2945278.
Article7. Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, Hung SC. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011; 117:459–469. DOI: 10.1182/blood-2010-05-287508. PMID: 20952688.
Article8. Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007; 11:335–347. DOI: 10.1016/j.ccr.2007.02.006. PMID: 17418410. PMCID: PMC3145406.
Article9. Zhang H, Nan W, Song X, Wang S, Si H, Li G. Knockdown of HIF-1α inhibits the proliferation and migration of outer root sheath cells exposed to hypoxia in vitro: an involvement of Shh pathway. Life Sci. 2017; 191:82–89. DOI: 10.1016/j.lfs.2017.10.011. PMID: 29030089.
Article10. Bader AM, Klose K, Bieback K, Korinth D, Schneider M, Seifert M, Choi YH, Kurtz A, Falk V, Stamm C. Hypoxic preconditioning increases survival and pro-angiogenic capacity of human cord blood mesenchymal stromal cells in vitro. PLoS One. 2015; 10:e0138477. DOI: 10.1371/journal.pone.0138477. PMID: 26380983. PMCID: PMC4575058.
Article11. Kim J, Ma T. Autocrine fibroblast growth factor 2-mediated interactions between human mesenchymal stem cells and the extracellular matrix under varying oxygen tension. J Cell Biochem. 2013; 114:716–727. DOI: 10.1002/jcb.24413. PMID: 23060043.
Article12. Qin HH, Filippi C, Sun S, Lehec S, Dhawan A, Hughes RD. Hypoxic preconditioning potentiates the trophic effects of mesenchymal stem cells on co-cultured human primary hepatocytes. Stem Cell Res Ther. 2015; 6:237. DOI: 10.1186/s13287-015-0218-7. PMID: 26626568. PMCID: PMC4667488.
Article13. Hoshikawa M, Ohbayashi N, Yonamine A, Konishi M, Ozaki K, Fukui S, Itoh N. Structure and expression of a novel fibroblast growth factor, FGF-17, preferentially expressed in the embryonic brain. Biochem Biophys Res Commun. 1998; 244:187–191. DOI: 10.1006/bbrc.1998.8239. PMID: 9514906.
Article14. Granerus M, Engström W. Dual effects of four members of the fibroblast growth factor member family on multiplication and motility in human teratocarcinoma cells in vitro. Anticancer Res. 2000; 20:3527–3531. PMID: 11131657.15. Nezu M, Tomonaga T, Sakai C, Ishii A, Itoga S, Nishimura M, Matsuo Y, Tagawa M, Nomura F. Expression of the fetal-oncogenic fibroblast growth factor-8/17/18 subfamily in human hematopoietic tumors. Biochem Biophys Res Commun. 2005; 335:843–849. DOI: 10.1016/j.bbrc.2005.07.153. PMID: 16095560.
Article16. Fortin D, Rom E, Sun H, Yayon A, Bansal R. Distinct fibroblast growth factor (FGF)/FGF receptor signaling pairs initiate diverse cellular responses in the oligodendrocyte lineage. J Neurosci. 2005; 25:7470–7479. DOI: 10.1523/JNEUROSCI.2120-05.2005. PMID: 16093398.
Article17. Kwon S, Ki SM, Park SE, Kim MJ, Hyung B, Lee NK, Shim S, Choi BO, Na DL, Lee JE, Chang JW. Anti-apoptotic effects of human Wharton’s jelly-derived mesenchymal stem cells on skeletal muscle cells mediated via secretion of XCL1. Mol Ther. 2016; 24:1550–1560. DOI: 10.1038/mt.2016.125. PMID: 27434589. PMCID: PMC5113102.
Article18. Mammone T, Gan D, Foyouzi-Youssefi R. Apoptotic cell death increases with senescence in normal human dermal fibroblast cultures. Cell Biol Int. 2006; 30:903–909. DOI: 10.1016/j.cellbi.2006.06.010. PMID: 16904918.
Article19. Kim M, Rhee JK, Choi H, Kwon A, Kim J, Lee GD, Jekarl DW, Lee S, Kim Y, Kim TM. Passage-dependent accumulation of somatic mutations in mesenchymal stromal cells during in vitro culture revealed by whole genome sequencing. Sci Rep. 2017; 7:14508. DOI: 10.1038/s41598-017-15155-5. PMID: 29109420. PMCID: PMC5674020.
Article20. Crowder SW, Horton LW, Lee SH, McClain CM, Hawkins OE, Palmer AM, Bae H, Richmond A, Sung HJ. Passage-dependent cancerous transformation of human mesenchymal stem cells under carcinogenic hypoxia. FASEB J. 2013; 27:2788–2798. DOI: 10.1096/fj.13-228288. PMID: 23568779. PMCID: PMC3688746.
Article21. Fernandes H, Dechering K, van Someren E, Steeghs I, Apotheker M, Mentink A, van Blitterswijk C, de Boer J. Effect of chordin-like 1 on MC3T3-E1 and human mesenchymal stem cells. Cells Tissues Organs. 2010; 191:443–452. DOI: 10.1159/000281825. PMID: 20130390.
Article22. Yun SP, Lee MY, Ryu JM, Song CH, Han HJ. Role of HIF-1alpha and VEGF in human mesenchymal stem cell proliferation by 17beta-estradiol: involvement of PKC, PI3K/Akt, and MAPKs. Am J Physiol Cell Physiol. 2009; 296:C317–C326. DOI: 10.1152/ajpcell.00415.2008. PMID: 18987249.23. Cheng GS, Zhang YS, Zhang TT, He L, Wang XY. Bone marrow-derived mesenchymal stem cells modified with IGFBP-3 inhibit the proliferation of pulmonary artery smooth muscle cells. Int J Mol Med. 2017; 39:223–230. DOI: 10.3892/ijmm.2016.2820. PMID: 27959432.
Article24. Hofmann Bowman M, Wilk J, Heydemann A, Kim G, Rehman J, Lodato JA, Raman J, McNally EM. S100A12 mediates aortic wall remodeling and aortic aneurysm. Circ Res. 2010; 106:145–154. DOI: 10.1161/CIRCRESAHA.109.209486. PMID: 19875725. PMCID: PMC2878187.
Article25. Kunath T, Saba-El-Leil MK, Almousailleakh M, Wray J, Meloche S, Smith A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007; 134:2895–2902. DOI: 10.1242/dev.02880. PMID: 17660198.
Article26. Conte C, Riant E, Toutain C, Pujol F, Arnal JF, Lenfant F, Prats AC. FGF2 translationally induced by hypoxia is involved in negative and positive feedback loops with HIF-1alpha. PLoS One. 2008; 3:e3078. DOI: 10.1371/journal.pone.0003078. PMID: 18728783. PMCID: PMC2518102.27. Yang J, Kim WJ, Jun HO, Lee EJ, Lee KW, Jeong JY, Lee SW. Hypoxia-induced fibroblast growth factor 11 stimulates capillary-like endothelial tube formation. Oncol Rep. 2015; 34:2745–2751. DOI: 10.3892/or.2015.4223. PMID: 26323829.
Article28. Zhang Q, Doucet M, Tomlinson RE, Han X, Quarles LD, Collins MT, Clemens TL. The hypoxia-inducible factor-1α activates ectopic production of fibroblast growth factor 23 in tumor-induced osteomalacia. Bone Res. 2016; 4:16011. DOI: 10.1038/boneres.2016.11. PMID: 27468359. PMCID: PMC4948305.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nervonic Acid Inhibits Replicative Senescence of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells
- Cardioprotective Effects of Wharton Jelly Derived Mesenchymal Stem Cell Transplantation in a Rodent Model of Myocardial Injury
- Cryopreservation of Human Wharton's Jelly-derived Mesenchymal Stem Cells Following Controlled Rate Freezing Protocol Using Different Cryoprotectants; A Comparative Study
- Comparative Evaluation for Potential Differentiation of Endothelial Progenitor Cells and Mesenchymal Stem Cells into Endothelial-Like Cells
- A study of growth factors, chondrogenic differentiation of mesenchymal stem cells and cell response by needle size differences in vitro