J Vet Sci.
2016 Jun;17(2):127-136. 10.4142/jvs.2016.17.2.127.
Effects of aluminum on the reduction of neural stem cells, proliferating cells, and differentiating neuroblasts in the dentate gyrus of D-galactose-treated mice via increasing oxidative stress
- Affiliations
-
- 1Department of Anatomy and Cell Biology, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea. ysyoon@snu.ac.kr
- 2BK21 PLUS Program for Creative Veterinary Science Research, Research Institute for Veterinary Science, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea.
- 3Department of Anatomy, College of Veterinary Medicine, Kangwon National University, Chuncheon 24341, Korea.
- 4Korea Mouse Phenotyping Center, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea.
- KMID: 2413161
- DOI: http://doi.org/10.4142/jvs.2016.17.2.127
Abstract
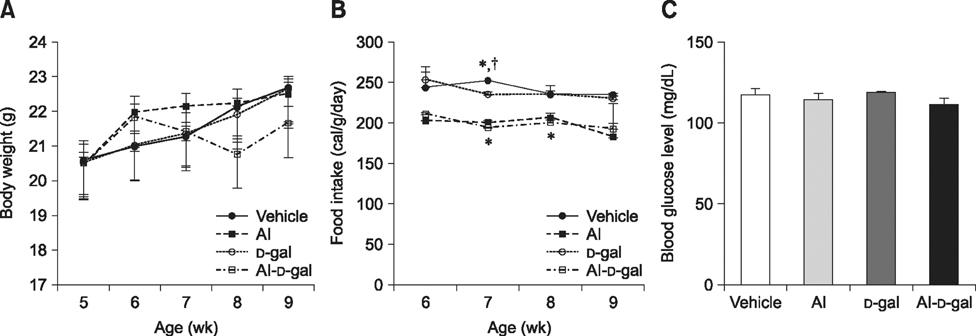
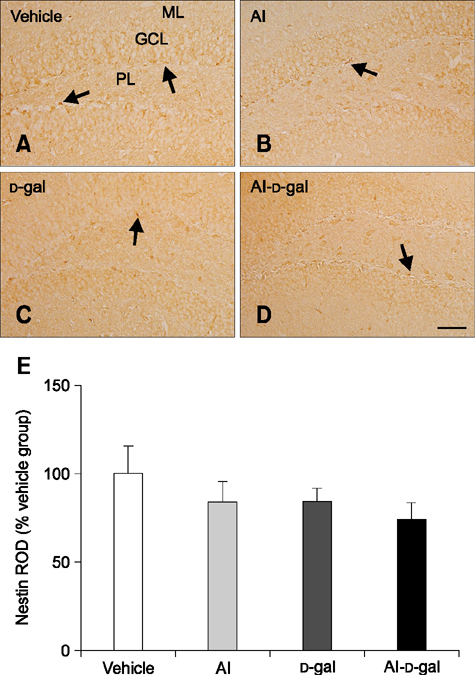
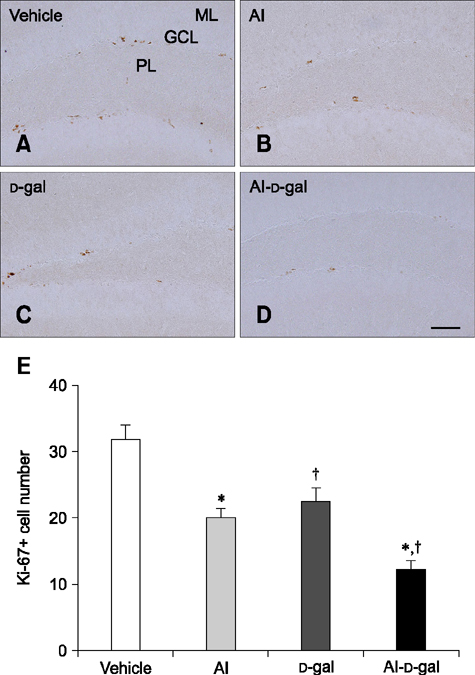
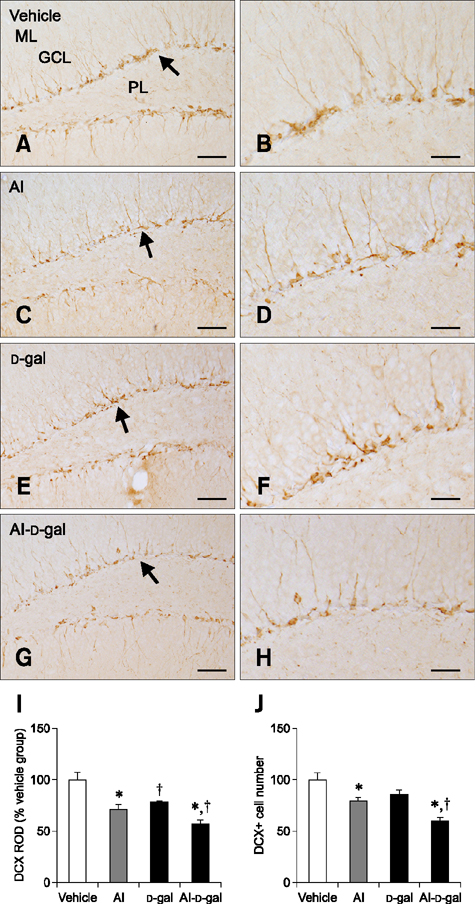
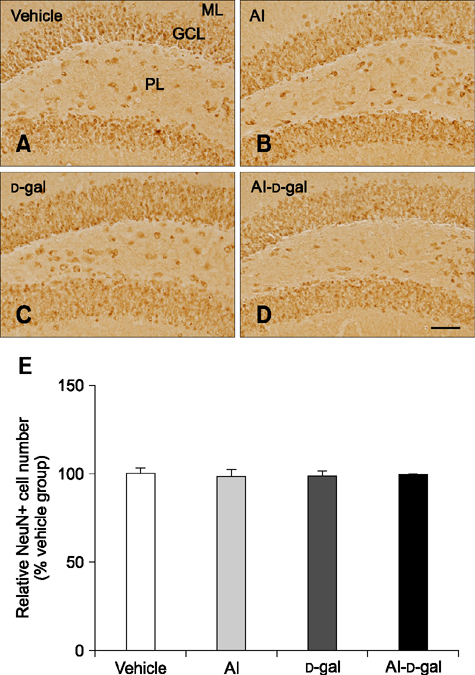
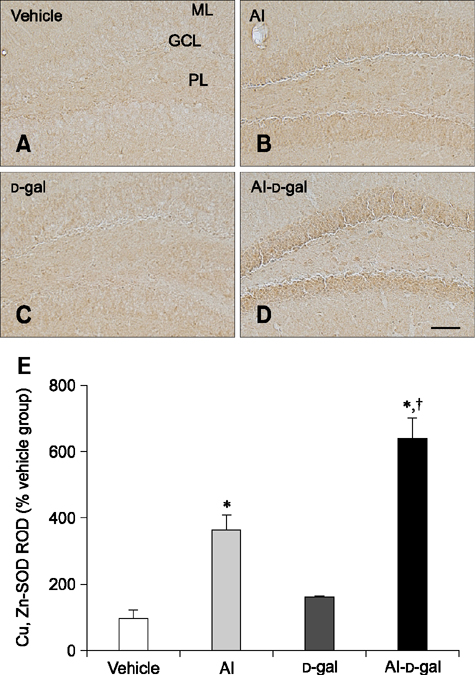
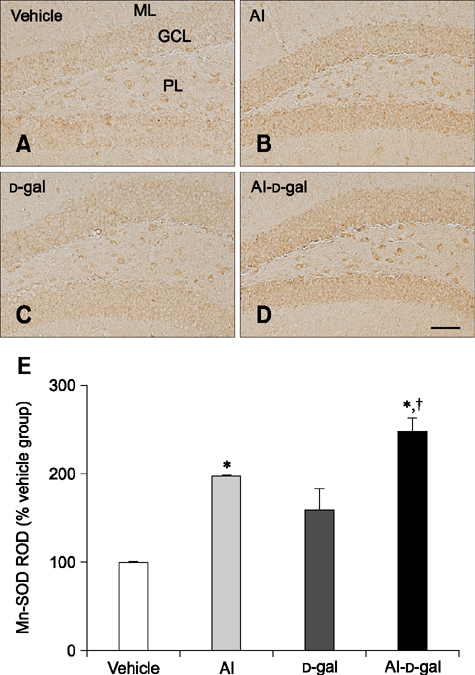
- Aluminum (Al) accumulation increases with aging, and long-term exposure to Al is regarded as a risk factor for Alzheimer's disease. In this study, we investigated the effects of Al and/or D-galactose on neural stem cells, proliferating cells, differentiating neuroblasts, and mature neurons in the hippocampal dentate gyrus. AlCl3 (40 mg/kg/day) was intraperitoneally administered to C57BL/6J mice for 4 weeks. In addition, vehicle (physiological saline) or D-galactose (100 mg/kg) was subcutaneously injected to these mice immediately after AlCl3 treatment. Neural stem cells, proliferating cells, differentiating neuroblasts, and mature neurons were detected using the relevant marker for each cell type, including nestin, Ki67, doublecortin, and NeuN, respectively, via immunohistochemistry. Subchronic (4 weeks) exposure to Al in mice reduced neural stem cells, proliferating cells, and differentiating neuroblasts without causing any changes to mature neurons. This Al-induced reduction effect was exacerbated in D-galactose-treated mice compared to vehicle-treated adult mice. Moreover, exposure to Al enhanced lipid peroxidation in the hippocampus and expression of antioxidants such as Cu, Zn- and Mn-superoxide dismutase in D-galactose-treated mice. These results suggest that Al accelerates the reduction of neural stem cells, proliferating cells, and differentiating neuroblasts in D-galactose-treated mice via oxidative stress, without inducing loss in mature neurons.
Keyword
MeSH Terms
-
Aluminum/*toxicity
Animals
Antioxidants/metabolism
Cell Differentiation/drug effects
Cell Proliferation/drug effects
Dentate Gyrus/*drug effects/physiology
Galactose/*toxicity
Hippocampus/drug effects/enzymology
Injections, Intraperitoneal
Injections, Subcutaneous
Lipid Peroxidation/drug effects
Male
Mice
Mice, Inbred C57BL
Neural Stem Cells/*drug effects/physiology
Neurogenesis/*drug effects
Neurons/*drug effects/physiology
Antioxidants
Aluminum
Galactose
Figure
Reference
-
1. Abdel Moneim AE, Othman MS, Mohmoud SM, El-Deib KM. Pomegranate peel attenuates aluminum-induced hepatorenal toxicity. Toxicol Mech Methods. 2013; 23:624–633.
Article2. Ansari MA, Scheff SW. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol. 2010; 69:155–167.
Article3. Ball MJ, Fisman M, Hachinski V, Blume W, Fox A, Kral VA, Kirshen AJ, Fox H, Merskey H. A new definition of Alzheimer's disease: a hippocampal dementia. Lancet. 1985; 1:14–16.
Article4. Bignucolo A, Lemire J, Auger C, Castonguay Z, Appanna V, Appanna VD. The molecular connection between aluminum toxicity, anemia, inflammation and obesity: therapeutic cues. In : Silverberg D, editor. Anemia. Rijeka: InTech;2012. p. 403–424.5. Crapo JD, Oury T, Rabouille C, Slot JW, Chang LY. Copper, zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc Natl Acad Sci U S A. 1992; 89:10405–10409.
Article6. Cui X, Zuo P, Zhang Q, Li X, Hu Y, Long J, Packer L, Liu J. Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-α-lipoic acid. J Neurosci Res. 2006; 84:647–654.
Article7. Eisenreich SJ. Atmospheric input of trace metals to Lake Michigan. Water Air Soil Pollut. 1980; 13:287–301.
Article8. Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010; 65:7–19.
Article9. Filipek LH, Nordstrom DK, Ficklin WH. Interaction of acid mine drainage with waters and sediments of West Squaw Creek in the West Shasta Mining District, California. Environ Sci Technol. 1987; 21:388–396.
Article10. Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press;1997.11. Freitas RM. Investigation of oxidative stress involvement in hippocampus in epilepsy model induced by pilocarpine. Neurosci Lett. 2009; 462:225–229.
Article12. Ganrot PO. Metabolism and possible health effects of aluminum. Environ Health Perspect. 1986; 65:363–441.
Article13. Gauthier E, Fortier I, Courchesne F, Pepin P, Mortimer J, Gauvreau D. Aluminum forms in drinking water and risk of Alzheimer's disease. Environ Res. 2000; 84:234–246.
Article14. Gómez M, Esparza JL, Cabré M, García T, Domingo JL. Aluminum exposure through the diet: metal levels in AβPP transgenic mice, a model for Alzheimer's disease. Toxicology. 2008; 249:214–219.
Article15. Grassie C, Braithwaite VA, Nilsson J, Nilsen TO, Teien HC, Handeland SO, Stefansson SO, Tronci V, Gorissen M, Flik G, Ebbesson LO. Aluminum exposure impacts brain plasticity and behavior in Atlantic salmon (Salmo salar). J Exp Biol. 2013; 216:3148–3155.
Article16. Grillo CA, Piroli GG, Rosell DR, Hoskin EK, Mcewen BS, Reagan LP. Region specific increases in oxidative stress and superoxide dismutase in the hippocampus of diabetic rats subjected to stress. Neuroscience. 2003; 121:133–140.
Article17. Harris WR, Berthon G, Day JP, Exley C, Flaten TP, Forbes WF, Kiss T, Orvig C, Zatta PF. Speciation of aluminum in biological systems. J Toxicol Environ Health. 1996; 48:543–568.
Article18. Hsieh HM, Wu WM, Hu ML. Soy isoflavones attenuate oxidative stress and improve parameters related to aging and Alzheimer's disease in C57BL/6J mice treated with D-galactose. Food Chem Toxicol. 2009; 47:625–632.
Article19. Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984; 225:1168–1170.
Article20. Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, Harry J, Kacew S, Lindsay J, Mahfouz AM, Rondeau V. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health B Crit Rev. 2007; 10:Suppl 1. 1–269.
Article21. Kruman I, Bruce-Keller AJ, Bredesen D, Waeg G, Mattson MP. Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. J Neurosci. 1997; 17:5089–5100.
Article22. Lei M, Hua X, Xiao M, Ding J, Han Q, Hu G. Impairments of astrocytes are involved in the D-galactose-induced brain aging. Biochem Biophys Res Commun. 2008; 369:1082–1087.
Article23. Lisman J. Formation of the non-functional and functional pools of granule cells in the dentate gyrus: role of neurogenesis, LTP and LTD. J Physiol. 2011; 589:1905–1909.
Article24. Lu J, Zheng YL, Wu DM, Luo L, Sun DX, Shan Q. Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by D-galactose. Biochem Pharmacol. 2007; 74:1078–1090.
Article25. Mićić DV, Petronijević ND, Vucetić SS. Superoxide dismutase activity in the mongolian gerbil brain after acute poisoning with aluminum. J Alzheimers Dis. 2003; 5:49–56.
Article26. Nam SM, Choi JH, Yoo DY, Kim W, Jung HY, Kim JW, Kang SY, Park J, Kim DW, Kim WJ, Yoon YS, Hwang IK. Valeriana officinalis extract and its main component, valerenic acid, ameliorate D-galactose-induced reductions in memory, cell proliferation, and neuroblast differentiation by reducing corticosterone levels and lipid peroxidation. Exp Gerontol. 2013; 48:1369–1377.
Article27. Nam SM, Kim JW, Yoo DY, Jung HY, Choi JH, Hwang IK, Seong JK, Yoon YS. Reduction of adult hippocampal neurogenesis is amplified by aluminum exposure in a model of type 2 diabetes. J Vet Sci. 2016; 17:13–20.
Article28. Nam SM, Kim JW, Yoo DY, Kim W, Jung HY, Hwang IK, Seong JK, Yoon YS. Additive or synergistic effects of aluminum on the reduction of neural stem cells, cell proliferation, and neuroblast differentiation in the dentate gyrus of high-fat diet-fed mice. Biol Trace Elem Res. 2014; 157:51–59.
Article29. Nayak P. Aluminum: impacts and disease. Environ Res. 2002; 89:101–115.
Article30. Poirier J, Semple H, Davies J, Lapointe R, Dziwenka M, Hiltz M, Mujibi D. Double-blind, vehicle-controlled randomized twelve-month neurodevelopmental toxicity study of common aluminum salts in the rat. Neuroscience. 2011; 193:338–362.
Article31. Priest ND. The biological behaviour and bioavailability of aluminium in man, with special reference to studies employing aluminium-26 as a tracer: review and study update. J Environ Monit. 2004; 6:375–403.
Article32. Salmon AB, Richardson A, Pérez VI. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med. 2010; 48:642–655.
Article33. Savory J, Herman MM, Ghribi O. Mechanisms of aluminum-induced neurodegeneration in animals: implications for Alzheimer's disease. J Alzheimers Dis. 2006; 10:135–144.
Article34. Song X, Bao M, Li D, Li YM. Advanced glycation in D-galactose induced mouse aging model. Mech Ageing Dev. 1999; 108:239–251.35. Southorn PA, Powis G. Free radicals in medicine. I. Chemical nature and biologic reactions. Mayo Clin Proc. 1988; 63:381–389.
Article36. Venkateshappa C, Harish G, Mahadevan A, Srinivas Bharath MM, Shankar SK. Elevated oxidative stress and decreased antioxidant function in the human hippocampus and frontal cortex with increasing age: implications for neurodegeneration in Alzheimer's disease. Neurochem Res. 2012; 37:1601–1614.
Article37. Walton JR. Brain lesions comprised of aluminum-rich cells that lack microtubules may be associated with the cognitive deficit of Alzheimer's disease. Neurotoxicology. 2009; 30:1059–1069.
Article38. Weisiger RA, Fridovich I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973; 248:4793–4796.39. Xiao F, Li XG, Zhang XY, Hou JD, Lin LF, Gao Q, Luo HM. Combined administration of D-galactose and aluminium induces Alzheimer-like lesions in brain. Neurosci Bull. 2011; 27:143–155.
Article40. Xie CX, Mattson MP, Lovell MA, Yokel RA. Intraneuronal aluminum potentiates iron-induced oxidative stress in cultured rat hippocampal neurons. Brain Res. 1996; 743:271–277.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Polarized and Stage-Dependent Distribution of Immunoreactivity for Novel PDZ-Binding Protein Preso1 in Adult Neurogenic Regions
- Comparison of pharmacological and genetic inhibition of cyclooxygenase-2: effects on adult neurogenesis in the hippocampal dentate gyrus
- Adult Neurogenesis in the Central and Peripheral Nervous Systems
- Neural Stem Cells and Ischemic Brain
- Toll-like receptor 2 promotes neurogenesis from the dentate gyrus after photothrombotic cerebral ischemia in mice