J Vet Sci.
2017 Mar;18(1):95-99. 10.4142/jvs.2017.18.1.95.
Probiotic properties and adsorption of Enterococcus faecalis PSCT3-7 to vermiculite
- Affiliations
-
- 1Laboratory of Veterinary Pharmacokinetics and Pharmacodynamics, College of Veterinary Medicine, Kyungpook National University, Daegu 41566, Korea. parksch@knu.ac.kr
- 2Lovelace Respiratory Research Institute, Albuquerque, NM 87108, USA.
- 3Laboratory of Veterinary Toxicology, College of Veterinary Medicine, Chonnam National University, Gwangju 61186, Korea.
- 4Department of Food Science and Technology, Keimyung University, Daegu 42601, Korea.
- 5Center for Nutraceutical and Pharmaceutical Materials, Department of Bioscience and Bioinformatics, Myongji University, Yongin 17058, Korea. parksch@knu.ac.kr
- KMID: 2412593
- DOI: http://doi.org/10.4142/jvs.2017.18.1.95
Abstract
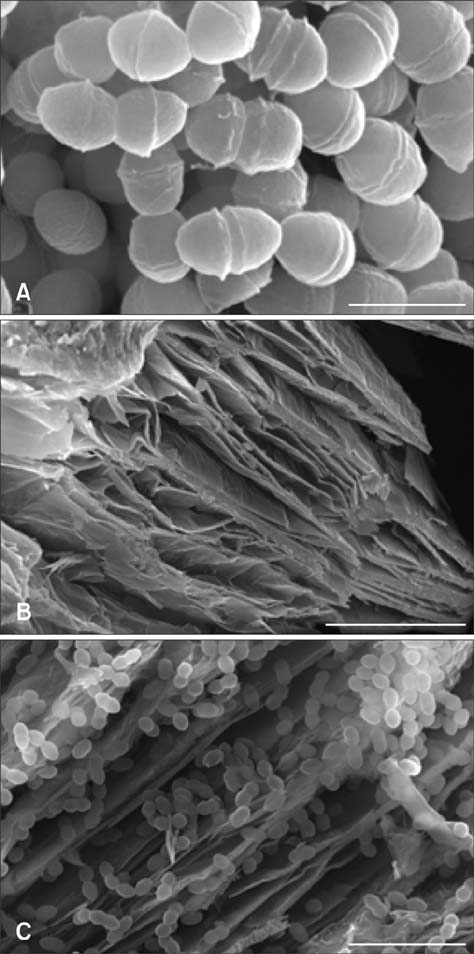
- The probiotic properties of Enterococcus (E.) faecalis PSCT3-7, a new strain isolated from the intestines of pigs fed dietary fiber containing 50% sawdust, were investigated. E. faecalis PSCT3-7 tolerated a pH range of 3 to 8 and 0.3% bile salts, and it inhibited the growth of Salmonella Typhimurium in a concentration-dependent manner. In addition, E. faecalis showed resistance to several antibacterial agents. Vermiculite, a nutrient and microbial carrier, increased the bile tolerance of the strain. Scanning electron microscope images revealed good adsorption of E. faecalis PSCT3-7 onto vermiculite. E. faecalis PSCT3-7 represents a potential probiotic candidate to administer with vermiculite to swine.
MeSH Terms
-
Adsorption
Aluminum Silicates/*chemistry
Animal Feed/analysis
Anti-Bacterial Agents/pharmacology
Diet/veterinary
Enterococcus faecalis/chemistry/drug effects/genetics/*physiology
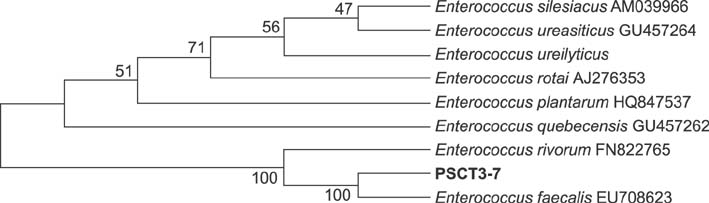
Phylogeny
Probiotics/administration & dosage/*chemistry/*pharmacology
RNA, Bacterial/genetics
RNA, Ribosomal, 16S/genetics
Aluminum Silicates
Anti-Bacterial Agents
RNA, Bacterial
RNA, Ribosomal, 16S
Figure
Reference
-
1. Anugwa FO, Varel VH, Dickson JS, Pond WG, Krook LP. Effects of dietary fiber and protein concentration on growth, feed efficiency, visceral organ weights and large intestine microbial populations of swine. J Nutr. 1989; 119:879–886.
Article2. Bederska-Łojewska D, Pieszka M. Modulating gastrointestinal Microflora of pigs through nutrition using feed additives. Ann Anim Sci. 2011; 11:333–335.3. Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005; 29:625–651.
Article4. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. Fifteenth informational supplement M100-S15. Wayne: CLSI;2005.5. England LS, Lee H, Trevors JT. Bacterial survival in soil: effect of clays and protozoa. Soil Biol Biochem. 1993; 25:525–531.
Article6. Forestier C, De Champs C, Vatoux C, Joly B. Probiotic activities of Lactobacillus casei rhamnosus: in vitro adherence to intestinal cells and antimicrobial properties. Res Microbiol. 2001; 152:167–173.
Article7. Franz CM, Van Belkum MJ, Holzapfel WH, Abriouel H, Gálvez A. Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol Rev. 2007; 31:293–310.
Article8. Gueimonde M, Sánchez B, de Los Reyes-Gavilán CG, Margolles A. Antibiotic resistance in probiotic bacteria. Front Microbiol. 2013; 4:202.
Article9. Kim EY, Kim YH, Rhee MH, Song JC, Lee KW, Kim KS, Lee SP, Lee IS, Park SC. Selection of Lactobacillus sp. PSC101 that produces active dietary enzymes such as amylase, lipase, phytase and protease in pigs. J Gen Appl Microbiol. 2007; 53:111–117.
Article10. Lee JS, Awji EG, Lee SJ, Tassew DD, Park YB, Park KS, Kim MK, Kim B, Park SC. Effect of Lactobacillus plantarum CJLP243 on the growth performance and cytokine response of weaning pigs challenged with enterotoxigenic Escherichia coli. J Anim Sci. 2012; 90:3709–3717.
Article11. Longland AC, Low AG, Quelch DB, Bray SP. Adaptation to the digestion of non-starch polysaccharide in growing pigs fed on cereal or semi-purified basal diets. Br J Nutr. 1993; 70:557–566.
Article12. Molist F, de Segura AG, Gasa J, Hermes RG, Manzanill EG, Anguita M, Pérez JF. Effects of the insoluble and soluble dietary fibre on the physicochemical properties of digesta and the microbial activity in early weaned piglets. Anim Feed Sci Technol. 2009; 149:346–353.
Article13. Nueno-Palop C, Narbad A. Probiotic assessment of Enterococcus faecalis CP58 isolated from human gut. Int J Food Microbiol. 2011; 145:390–394.
Article14. Pesenti-Barili B, Ferdani E, Mosti M, Degli-Innocenti F. Survival of Agrobacterium radiobacter K84 on various carriers for crown gall control. Appl Environ Microbiol. 1991; 57:2047–2051.
Article15. Varel VH, Pond WG. Enumeration and activity of cellulolytic bacteria from gestating swine fed various levels of dietary fiber. Appl Environ Microbiol. 1985; 49:858–862.
Article16. Wellinghausen N, Chatterjee I, Berger A, Niederfuehr A, Proctor RA, Kahl BC. Characterization of clinical Enterococcus faecalis small-colony variants. J Clin Microbiol. 2009; 47:2802–2811.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Bilateral Endogenous Enterococcus Faecalis Endophthalmitis in Liver Abscess
- Recurrent Enterococcus faecalis Endophthalmitis
- Clinical Features and Rate of Infective Endocarditis in Non-Faecalis and Non-faecium Enterococcal Bacteremia
- The effect of using nanoparticles in bioactive glass on its antimicrobial properties
- A Case of Enterococcus Faecalis Endophthalmitis with Corneal Ulcer