Nutr Res Pract.
2017 Apr;11(2):83-89. 10.4162/nrp.2017.11.2.83.
Wheatgrass extract inhibits hypoxia-inducible factor-1-mediated epithelial-mesenchymal transition in A549 cells
- Affiliations
-
- 1Department of Otorhinolaryngology-Head & Neck Surgery, Chosun University College of Medicine, 365 Plimundaero, Dong-gu, Gwangju 61452, Korea. gracelee@chosun.ac.kr
- 2Department of Biochemical and Polymer Engineering, Chosun University, Gwangju 61452, Korea.
- KMID: 2412019
- DOI: http://doi.org/10.4162/nrp.2017.11.2.83
Abstract
- BACKGROUND/OBJECTIVES
Epithelial-mesenchymal transition (EMT) is involved in not only cancer development and metastasis but also non-cancerous conditions. Hypoxia is one of the proposed critical factors contributing to formation of chronic rhinosinusitis or nasal polyposis. Wheatgrass (Triticum aestivum) has antioxidant, anti-aging, and anti-inflammatory effects. In this study, we analyzed whether wheatgrass has an inhibitory effect on the EMT process in airway epithelial cells.
MATERIALS/METHODS
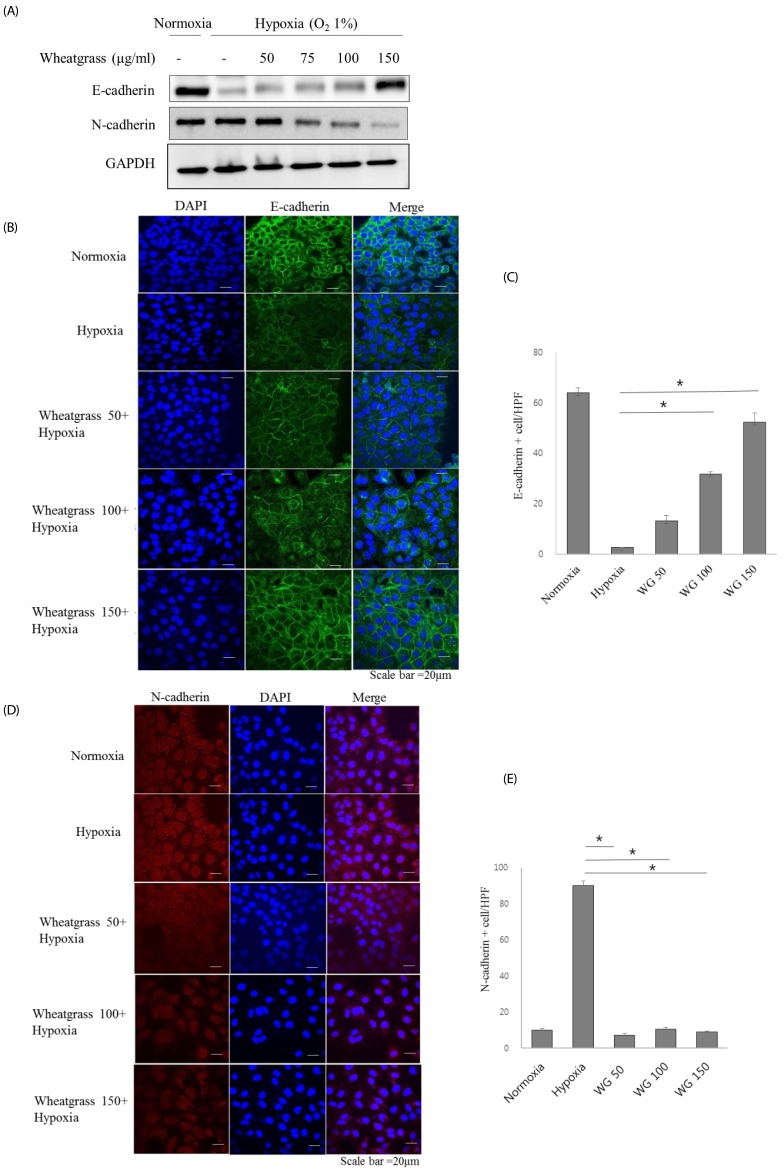
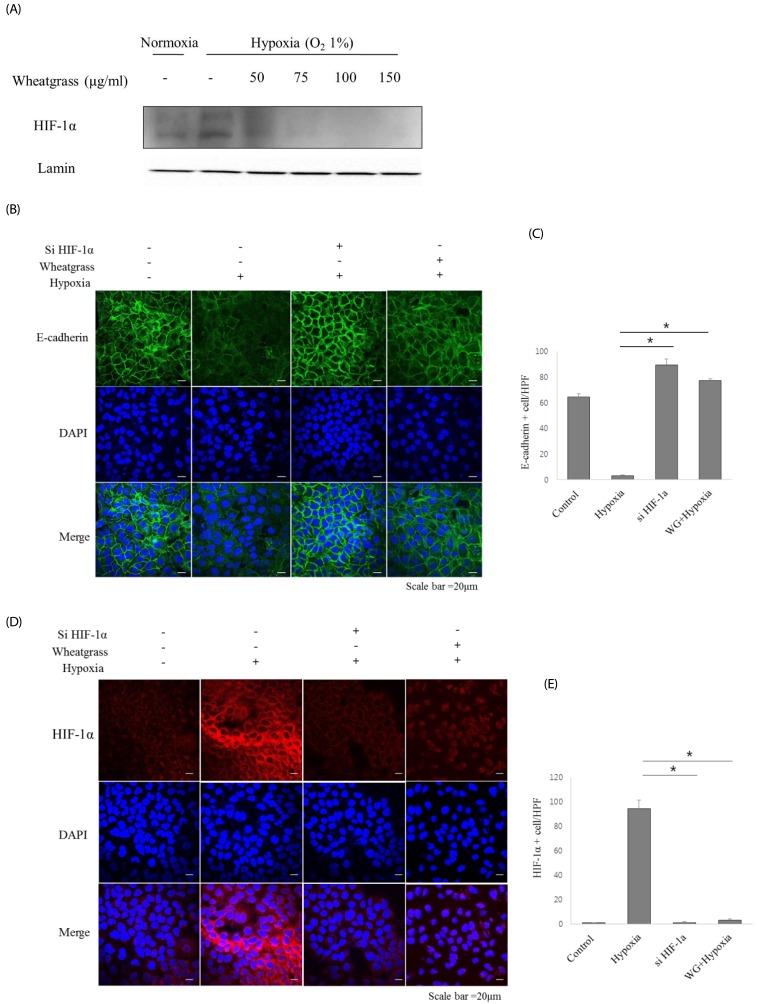
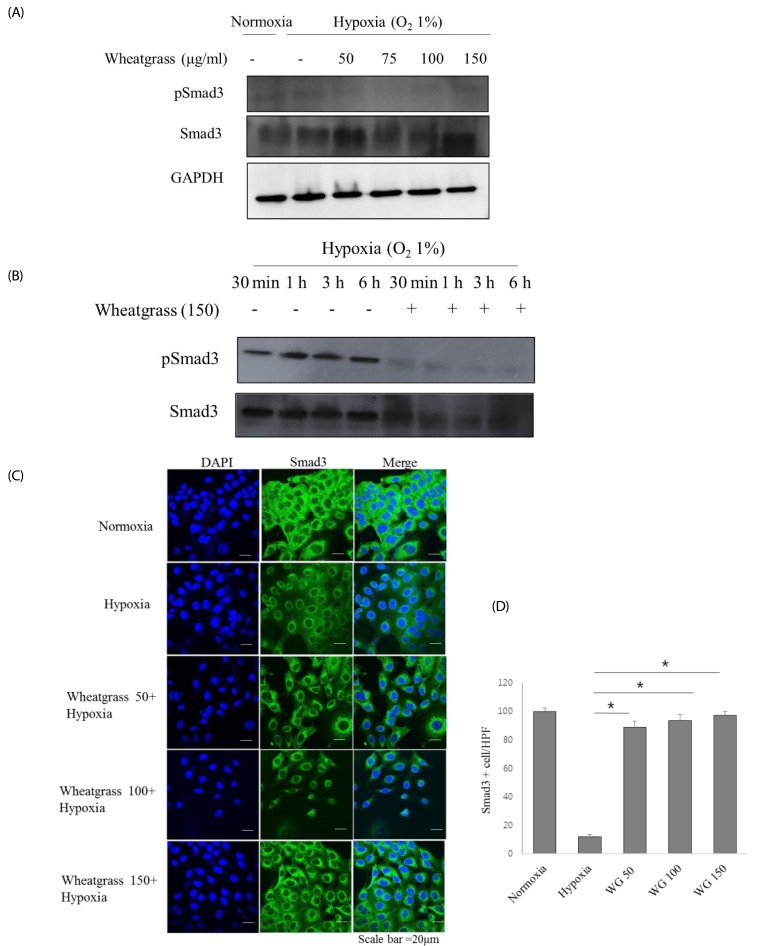
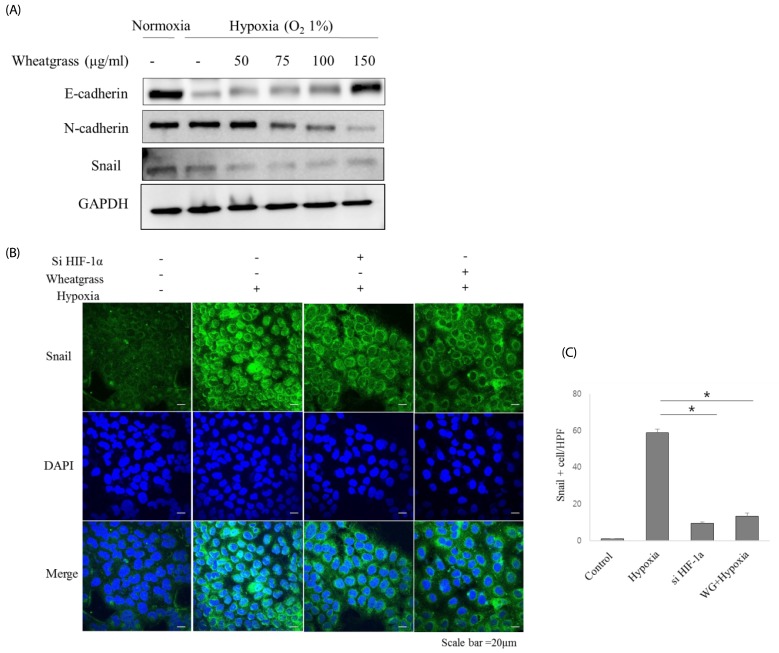
A549 human lung adenocarcinoma cells were incubated in hypoxic conditions (COâ‚‚ 5%/Oâ‚‚ 1%) for 24 h in the presence of different concentrations of wheatgrass extract (50, 75, 100, and 150 µg/mL) and changes in expression of epithelial or mesenchymal markers were evaluated by immunoblotting and immunofluorescence. Accordingly, associated EMT-related transcriptional factors, Snail and Smad, were also evaluated.
RESULTS
Hypoxia increased expression of N-cadherin and reduced expression of E-cadherin. Mechanistically, E-cadherin levels were recovered during hypoxia by silencing hypoxia inducible factor (HIF)-1α or administering wheatgrass extract. Wheatgrass inhibited the hypoxia-mediated EMT by reducing the expression of phosphorylated Smad3 (pSmad3) and Snail. It suppressed the hypoxia-mediated EMT processes of airway epithelial cells via HIF-1α and the pSmad3 signaling pathway.
CONCLUSION
These results suggest that wheatgrass has potential as a therapeutic or supplementary agent for HIF-1-related diseases.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Retraction: Wheatgrass extract inhibits hypoxia-inducible factor-1-mediated epithelial-mesenchymal transition in A549 cells
Nam Yong Do, Hyun-Jae Shin, Ji-Eun Lee
Nutr Res Pract. 2018;12(3):265-265. doi: 10.4162/nrp.2018.12.3.265.
Reference
-
1. Hsu YC, Kuo WR, Chen YY, Tai CF, Tsai CJ, Wang LF. Increased expression of hypoxia-inducible factor 1alpha in the nasal polyps. Am J Otolaryngol. 2007; 28:379–383. PMID: 17980768.2. Chien CY, Tai CF, Ho KY, Kuo WR, Chai CY, Hsu YC, Wang LF. Expression of hypoxia-inducible factor 1alpha in the nasal polyps by real-time RT-PCR and immunohistochemistry. Otolaryngol Head Neck Surg. 2008; 139:206–210. PMID: 18656716.3. Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006; 7:131–142. PMID: 16493418.
Article4. Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, Murray LA, Argentieri R, Kicic A, Stick SM, Bai TR, Knight DA. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med. 2009; 180:122–133. PMID: 19406982.5. Shin HW, Cho K, Kim DW, Han DH, Khalmuratova R, Kim SW, Jeon SY, Min YG, Lee CH, Rhee CS, Park JW. Hypoxia-inducible factor 1 mediates nasal polypogenesis by inducing epithelial-to-mesenchymal transition. Am J Respir Crit Care Med. 2012; 185:944–954. PMID: 22323302.
Article6. Alitheen NB, Oon CL, Keong YS, Chuan TK, Li HK, Yong HW. Cytotoxic effects of commercial wheatgrass and fiber towards human acute promyelocytic leukemia cells (HL60). Pak J Pharm Sci. 2011; 24:243–250. PMID: 21715255.7. Ferruzzi MG, Blakeslee J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr Res. 2007; 27:1–12.
Article8. Das A, Raychaudhuri U, Chakraborty R. Effect of freeze drying and oven drying on antioxidant properties of fresh wheatgrass. Int J Food Sci Nutr. 2012; 63:718–721. PMID: 22171655.
Article9. Kulkarni SD, Tilak JC, Acharya R, Rajurkar NS, Devasagayam TP, Reddy AV. Evaluation of the antioxidant activity of wheatgrass (Triticum aestivum L.) as a function of growth under different conditions. Phytother Res. 2006; 20:218–227. PMID: 16521113.
Article10. Ben-Arye E, Goldin E, Wengrower D, Stamper A, Kohn R, Berry E. Wheat grass juice in the treatment of active distal ulcerative colitis: a randomized double-blind placebo-controlled trial. Scand J Gastroenterol. 2002; 37:444–449. PMID: 11989836.
Article11. Shermer M. Wheatgrass juice and folk medicine. Sci Am. 2008; 299:42.
Article12. Steinke JW, Woodard CR, Borish L. Role of hypoxia in inflammatory upper airway disease. Curr Opin Allergy Clin Immunol. 2008; 8:16–20. PMID: 18188012.
Article13. Matsune S, Kono M, Sun D, Ushikai M, Kurono Y. Hypoxia in paranasal sinuses of patients with chronic sinusitis with or without the complication of nasal allergy. Acta Otolaryngol. 2003; 123:519–523. PMID: 12797588.
Article14. Lu X, Zhang XH, Wang H, Long XB, You XJ, Gao QX, Cui YH, Liu Z. Expression of osteopontin in chronic rhinosinusitis with and without nasal polyps. Allergy. 2009; 64:104–111. PMID: 19076536.
Article15. Shakya G, Balasubramanian S, Rajagopalan R. Methanol extract of wheatgrass induces G1 cell cycle arrest in a p53-dependent manner and down regulates the expression of cyclin D1 in human laryngeal cancer cells-an in vitro and in silico approach. Pharmacogn Mag. 2015; 11:S139–S147. PMID: 26109759.16. Chiu LC, Kong CK, Ooi VE. The chlorophyllin-induced cell cycle arrest and apoptosis in human breast cancer MCF-7 cells is associated with ERK deactivation and Cyclin D1 depletion. Int J Mol Med. 2005; 16:735–740. PMID: 16142413.17. Aydos SE, Avci A, Özkan T, Karadağ A, Gürleyik E, Altinok B, Sunguroğlu A. Antiproliferative, apoptotic and antioxidant activities of wheatgrass (Triticum aestivum L.) extract on CML (K562) cell line. Turk J Med Sci. 2011; 41:657–663.18. Lam CR, Brush BE. Chlorophyll and wound healing; experimental and clinical study. Am J Surg. 1950; 80:204–210. PMID: 15425708.19. Shakya G, Randhi PK, Pajaniradje S, Mohankumar K, Rajagopalan R. Hypoglycaemic role of wheatgrass and its effect on carbohydrate metabolic enzymes in type II diabetic rats. Toxicol Ind Health. 2016; 32:1026–1032. PMID: 25116122.
Article20. Jaya MS, Gayathri S. Antioxidant activity of wheat grass and impact of supplementing grass extract on anaemics. Biomed. 2009; 4:262–268.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retraction: Wheatgrass extract inhibits hypoxia-inducible factor-1-mediated epithelial-mesenchymal transition in A549 cells
- ACY-241, a histone deacetylase 6 inhibitor, suppresses the epithelial–mesenchymal transition in lung cancer cells by downregulating hypoxia-inducible factor-1 alpha
- Downregulation of TET2 Contributes to Nasal Polypogenesis Through Hypoxia-Inducible Factor 1α-Mediated Epithelial-to-Mesenchymal Transition
- Hypoxia Induces Epithelial-Mesenchymal Transition in Follicular Thyroid Cancer: Involvement of Regulation of Twist by Hypoxia Inducible Factor-1alpha
- Trefoil Factor 1 Suppresses Epithelial-mesenchymal Transition through Inhibition of TGF-beta Signaling in Gastric Cancer Cells