Immune Netw.
2018 Apr;18(2):e9. 10.4110/in.2018.18.e9.
Optimization of Cytokine Milieu to Reproduce Atopic Dermatitis-related Gene Expression in HaCaT Keratinocyte Cell Line
- Affiliations
-
- 1Department of Dermatology, Gachon Gil Medical Center, School of Medicine, Gachon University, Incheon 21565, Korea. jyroh1@gilhospital.com
- 2Department of Microbiology, School of Medicine, Gachon University, Incheon 21565, Korea. yjjung@gachon.ac.kr
- 3Gachon Advanced Institute for Health Science & Technology, School of Medicine, Gachon University, Incheon 21565, Korea.
- KMID: 2410159
- DOI: http://doi.org/10.4110/in.2018.18.e9
Abstract
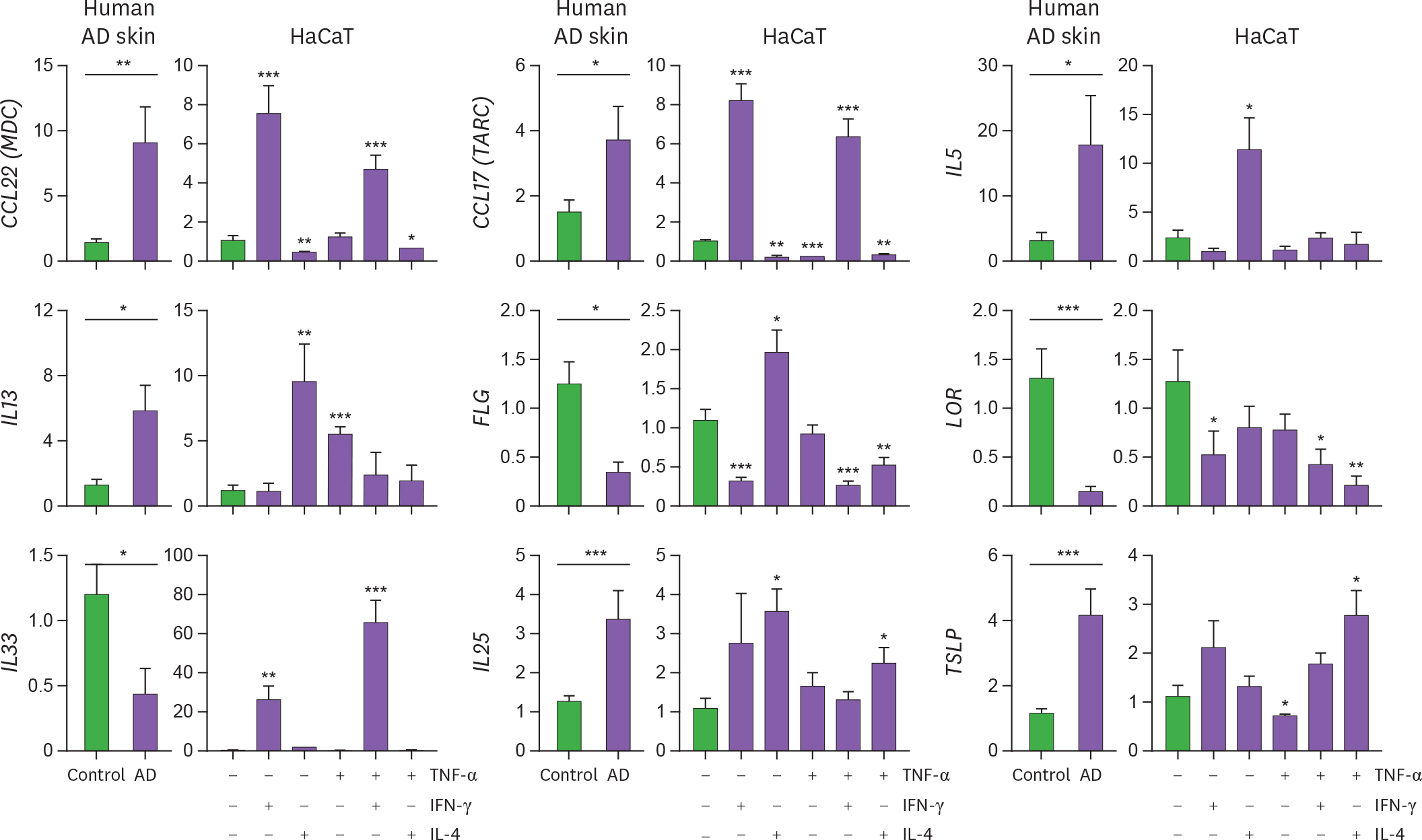
- Although atopic dermatitis (AD) is characterized by cytokine production predominantly mediated by T helper (Th) 2 cells, AD pathogenesis also involves innate immune and Th1 cells. To optimize the cytokine milieu required for accurate reproduction of AD-related gene expression profile in vitro, we evaluated the expression pattern of CCL22, CCL17, IL5, IL13, IL33, IL25, TSLP, FLG, and LOR in human lesional AD skin and cytokine-stimulated HaCaT cells. An increase in Th2 mediators (IL5, IL13, CCL22, CCL17, IL25, IL33, and TSLP) and a decrease in genes related to cornified cell envelope (filaggrin and loricrin) were observed in human AD lesions. Innate (tumor necrosis factor-α) and/or Th1/Th2 adaptive cytokines (interferon-γ/IL-4) were required for inducing these inflammatory changes in HaCaT cells, implying that a complex network of innate, Th1, and Th2 cytokines drives AD-like changes. Therefore, stimulation with various combinations of cytokines, beyond Th2 polarization, is necessary when HaCaT cell line is used to study genetic changes implicated in AD pathogenesis.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Hamid Q, Naseer T, Minshall EM, Song YL, Boguniewicz M, Leung DY. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol. 1996; 98:225–231.2. Leung DY. Pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 1999; 104:S99–S108.
Article3. Bartemes KR, Kita H. Dynamic role of epithelium-derived cytokines in asthma. Clin Immunol. 2012; 143:222–235.
Article4. Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST, McKenzie AN, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013; 210:2939–2950.
Article5. Grewe M, Gyufko K, Schöpf E, Krutmann J. Lesional expression of interferon-gamma in atopic eczema. Lancet. 1994; 343:25–26.6. Shimada Y, Takehara K, Sato S. Both Th2 and Th1 chemokines (TARC/CCL17, MDC/CCL22, and Mig/CXCL9) are elevated in sera from patients with atopic dermatitis. J Dermatol Sci. 2004; 34:201–208.
Article7. Guttman-Yassky E, Suárez-Fariñas M, Chiricozzi A, Nograles KE, Shemer A, Fuentes-Duculan J, Cardinale I, Lin P, Bergman R, Bowcock AM, et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J Allergy Clin Immunol. 2009; 124:1235–1244. e58.
Article8. Kim BE, Howell MD, Guttman-Yassky E, Gilleaudeau PM, Cardinale IR, Boguniewicz M, Krueger JG, Leung DY. TNF-α downregulates filaggrin and loricrin through c-Jun N-terminal kinase: role for TNF-α antagonists to improve skin barrier. J Invest Dermatol. 2011; 131:1272–1279.
Article9. Savinko T, Matikainen S, Saarialho-Kere U, Lehto M, Wang G, Lehtimäki S, Karisola P, Reunala T, Wolff H, Lauerma A, et al. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J Invest Dermatol. 2012; 132:1392–1400.
Article10. Omori-Miyake M, Yamashita M, Tsunemi Y, Kawashima M, Yagi J. In vitro assessment of IL-4- or IL-13-mediated changes in the structural components of keratinocytes in mice and humans. J Invest Dermatol. 2014; 134:1342–1350.11. Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, DeBenedetto A, Schneider L, Beck LA, Barnes KC, Leung DY. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2009; 124:R7–R12.
Article12. Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005; 23:479–490.
Article13. Kakinuma T, Nakamura K, Wakugawa M, Yano S, Saeki H, Torii H, Komine M, Asahina A, Tamaki K. IL-4, but not IL-13, modulates TARC (thymus and activation-regulated chemokine)/CCL17 and IP-10 (interferon-induced protein of 10kDA)/CXCL10 release by TNF-alpha and IFN-gamma in HaCaT cell line. Cytokine. 2002; 20:1–6.14. Xiao T, Kagami S, Saeki H, Sugaya M, Kakinuma T, Fujita H, Yano S, Mitsui H, Torii H, Komine M, et al. Both IL-4 and IL-13 inhibit the TNF-alpha and IFN-gamma enhanced MDC production in a human keratinocyte cell line, HaCaT cells. J Dermatol Sci. 2003; 31:111–117.15. Albanesi C, Scarponi C, Sebastiani S, Cavani A, Federici M, Sozzani S, Girolomoni G. A cytokine-to-chemokine axis between T lymphocytes and keratinocytes can favor Th1 cell accumulation in chronic inflammatory skin diseases. J Leukoc Biol. 2001; 70:617–623.16. Fort M, Lesley R, Davidson N, Menon S, Brombacher F, Leach M, Rennick D. IL-4 exacerbates disease in a Th1 cell transfer model of colitis. J Immunol. 2001; 166:2793–2800.
Article17. Jacobs MJ, van den Hoek AE, van Lent PL, van de Loo FA, van de Putte LB, van den Berg WB. Role of IL-2 and IL-4 in exacerbations of murine antigen-induced arthritis. Immunology. 1994; 83:390–396.18. Ramanathan S, de Kozak Y, Saoudi A, Goureau O, Van der Meide PH, Druet P, Bellon B. Recombinant IL-4 aggravates experimental autoimmune uveoretinitis in rats. J Immunol. 1996; 157:2209–2215.19. Danso MO, van Drongelen V, Mulder A, van Esch J, Scott H, van Smeden J, El Ghalbzouri A, Bouwstra JA. TNF-α and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J Invest Dermatol. 2014; 134:1941–1950.
Article20. Lehmann B. HaCaT cell line as a model system for vitamin D3 metabolism in human skin. J Invest Dermatol. 1997; 108:78–82.
Article21. Divekar R, Kita H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr Opin Allergy Clin Immunol. 2015; 15:98–103.
Article22. Cevikbas F, Steinhoff M. IL-33: a novel danger signal system in atopic dermatitis. J Invest Dermatol. 2012; 132:1326–1329.
Article23. Roy A, Ganesh G, Sippola H, Bolin S, Sawesi O, Dagälv A, Schlenner SM, Feyerabend T, Rodewald HR, Kjellén L, et al. Mast cell chymase degrades the alarmins heat shock protein 70, biglycan, HMGB1, and interleukin-33 (IL-33) and limits danger-induced inflammation. J Biol Chem. 2014; 289:237–250.
Article24. Seltmann J, Werfel T, Wittmann M. Evidence for a regulatory loop between IFN-γ and IL-33 in skin inflammation. Exp Dermatol. 2013; 22:102–107.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Adenosine on Enhanced ICAM-1 Expression by IFN-gamma in Cultured Human Keratinocyte Cell Line HaCaT Cells
- Expression and Modulation of LL-37 in Normal Human Keratinocytes, HaCaT cells, and Inflammatory Skin Diseases
- The Production IL-21 and VEGF in UVB-irradiated Human Keratinocyte Cell Line, HaCaT
- Development of In Vitro Co-Culture Model to Mimic the Cell to Cell Communication in Response to Urban PM2.5
- Adaptive Response and Apoptosis Induced by UV Irradiation in Human Keratinocytes and Squamous Cell Carcinomas