J Korean Med Sci.
2017 Oct;32(10):1616-1625. 10.3346/jkms.2017.32.10.1616.
Fate of Neutrophils during the Recovery Phase of Ischemia/Reperfusion Induced Acute Kidney Injury
- Affiliations
-
- 1Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. sang-kyung@korea.ac.kr
- 2Department of Radiation Cancer Science, Korea Institute of Radiological & Medical Sciences, Seoul, Korea.
- 3Department of Internal Medicine, Inje University College of Medicine, Seoul, Korea.
- KMID: 2400433
- DOI: http://doi.org/10.3346/jkms.2017.32.10.1616
Abstract
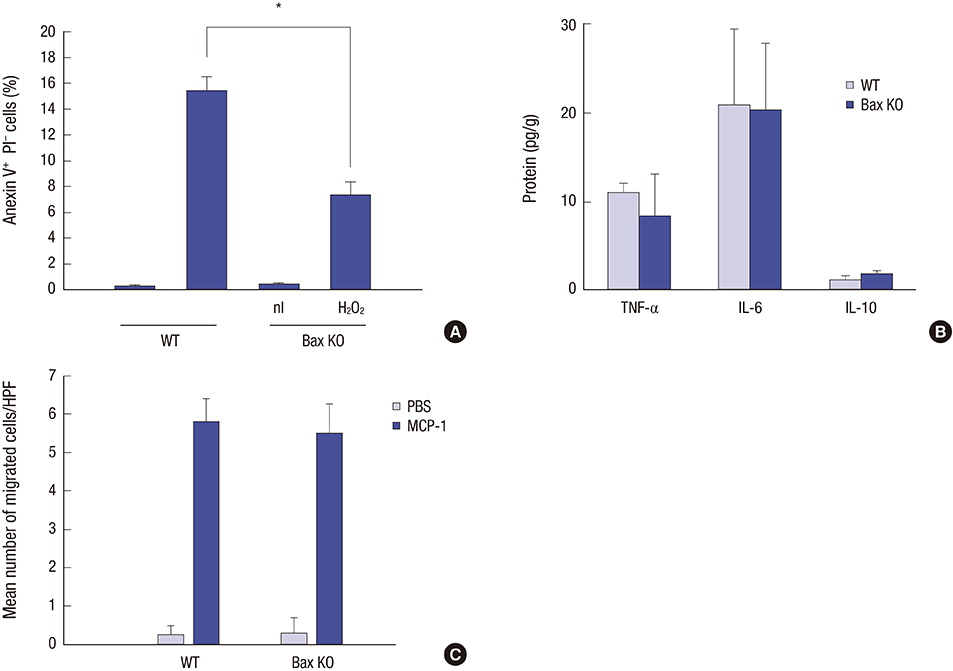
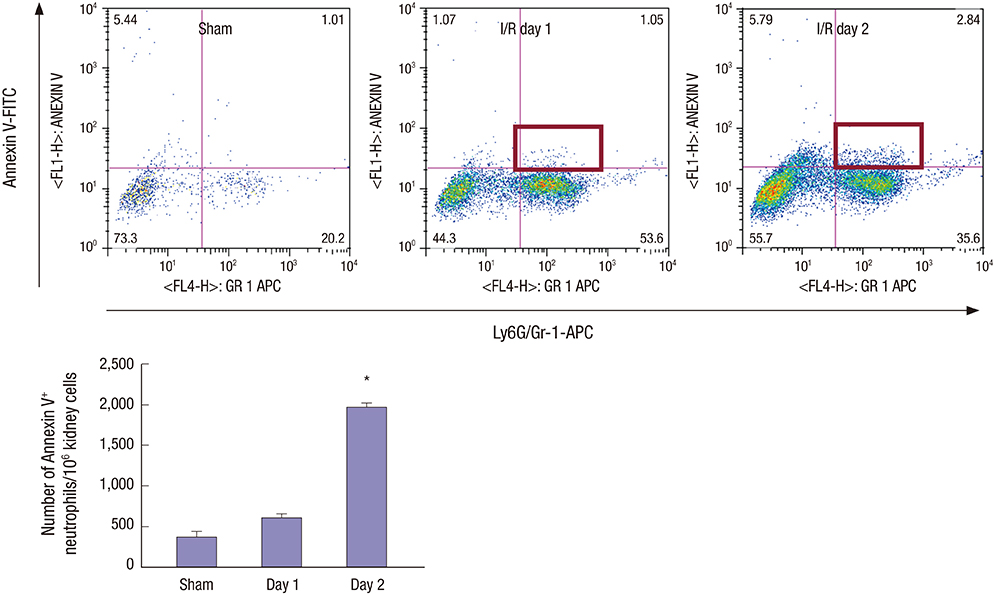
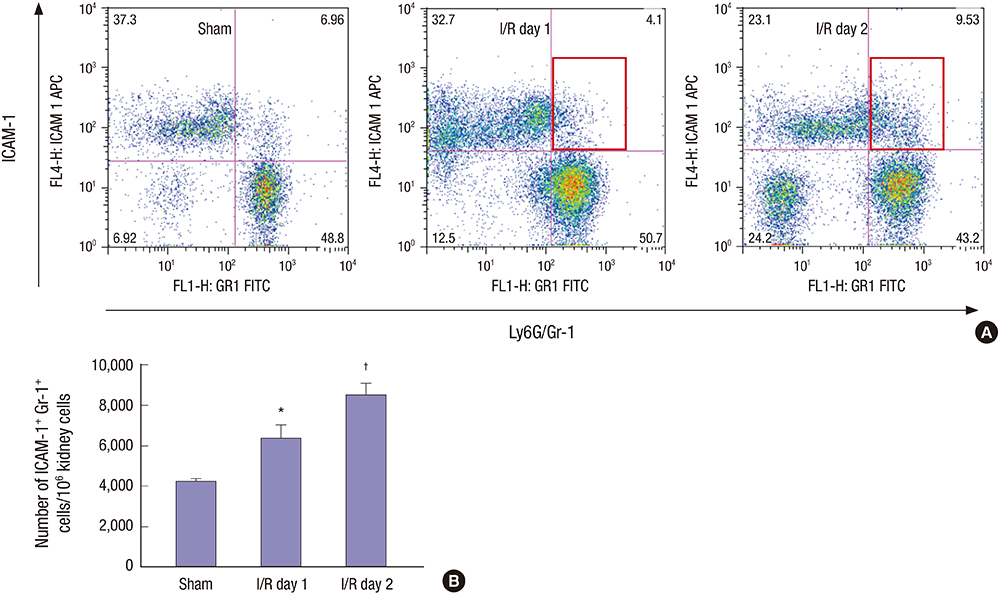
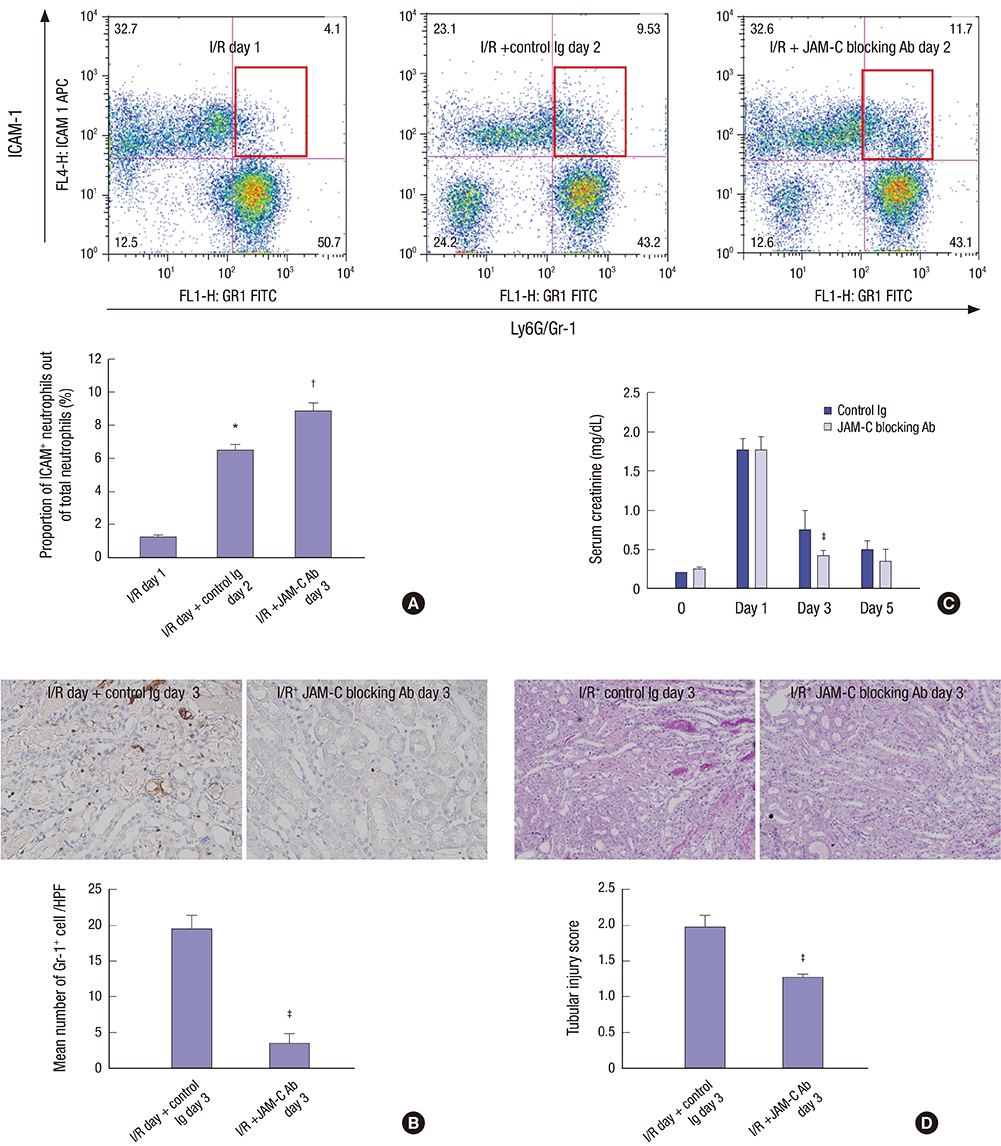
- Effective clearance of inflammatory cells is required for resolution of inflammation. Here, we show in vivo evidence that apoptosis and reverse transendothelial migration (rTEM) are important mechanisms in eliminating neutrophils and facilitating recovery following ischemia/reperfusion injury (IRI) of the kidney. The clearance of neutrophils was delayed in the Bax knockout (KO)BM → wild-type (WT) chimera in which bone marrow derived cells are partially resistant to apoptosis, compared to WTBM → WT mice. These mice also showed delayed functional, histological recovery, increased tissue cytokines, and accelerated fibrosis. The circulating intercellular adhesion molecule-1 (ICAM-1)+ Gr-1+ neutrophils displaying rTEM phenotype increased during the recovery phase and blockade of junctional adhesion molecule-C (JAM-C), a negative regulator of rTEM, resulted in an increase in circulating ICAM-1+ neutrophils, faster resolution of inflammation and recovery. The presence of Tamm-Horsfall protein (THP) in circulating ICAM-1+ neutrophils could suggest that they are derived from injured kidneys. In conclusion, we suggest that apoptosis and rTEM are critically involved in the clearance mechanisms of neutrophils during the recovery phase of IRI.
MeSH Terms
Figure
Reference
-
1. Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011; 121:4210–4221.2. Kelly KJ, Williams WW Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996; 97:1056–1063.3. Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol. 2011; 12:761–769.4. Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995; 270:96–99.5. Bagshaw SM, Laupland KB, Doig CJ, Mortis G, Fick GH, Mucenski M, Godinez-Luna T, Svenson LW, Rosenal T. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005; 9:R700–R709.6. Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008; 23:1203–1210.7. Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011; 22:317–326.8. Henson PM. Dampening inflammation. Nat Immunol. 2005; 6:1179–1181.9. Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007; 25:101–137.10. Saverymuttu SH, Peters AM, Keshavarzian A, Reavy HJ, Lavender JP. The kinetics of 111 indium distribution following injection of 111 indium labelled autologous granulocytes in man. Br J Haematol. 1985; 61:675–685.11. Wei Q, Dong G, Chen JK, Ramesh G, Dong Z. Bax and Bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney Int. 2013; 84:138–148.12. Dibbert B, Weber M, Nikolaizik WH, Vogt P, Schöni MH, Blaser K, Simon HU. Cytokine-mediated Bax deficiency and consequent delayed neutrophil apoptosis: a general mechanism to accumulate effector cells in inflammation. Proc Natl Acad Sci USA. 1999; 96:13330–13335.13. Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007; 9:550–555.14. Hanses F, Park S, Rich J, Lee JC. Reduced neutrophil apoptosis in diabetic mice during staphylococcal infection leads to prolonged Tnfα production and reduced neutrophil clearance. PLoS One. 2011; 6:e23633.15. Loynes CA, Martin JS, Robertson A, Trushell DM, Ingham PW, Whyte MK, Renshaw SA. Pivotal Advance: Pharmacological manipulation of inflammation resolution during spontaneously resolving tissue neutrophilia in the zebrafish. J Leukoc Biol. 2010; 87:203–212.16. Jo SK, Sung SA, Cho WY, Go KJ, Kim HK. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant. 2006; 21:1231–1239.17. Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, Huttenlocher A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol. 2006; 80:1281–1288.18. Buckley CD, Ross EA, McGettrick HM, Osborne CE, Haworth O, Schmutz C, Stone PC, Salmon M, Matharu NM, Vohra RK, et al. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J Leukoc Biol. 2006; 79:303–311.19. El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, Wu XR. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol. 2013; 304:F1066–F1075.20. Bradfield PF, Scheiermann C, Nourshargh S, Ody C, Luscinskas FW, Rainger GE, Nash GB, Miljkovic-Licina M, Aurrand-Lions M, Imhof BA. JAM-C regulates unidirectional monocyte transendothelial migration in inflammation. Blood. 2007; 110:2545–2555.21. Kim SC, Ko YS, Lee HY, Kim MG, Jo SK, Cho WY. Blocking junctional adhesion molecule C promotes the recovery of cisplatin-induced acute kidney injury. Korean J Intern Med. Forthcoming. 2017.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Kidney transplantation and ischemic conditioning: past, present and future perspectives
- Experimental Study on the Characteristics of Nerve Injury after Ischemia-Reperfusion and Their Recovery in Rats
- Mechanisms and therapeutic targets of ischemic acute kidney injury
- Does Heparin Attenuate the Renal Injury Induced by Ischemia Reperfusion in the Rabbit?
- Heparin attenuated neutrophil infiltration but did not affect renal injury induced by ischemia reperfusion