J Clin Neurol.
2009 Mar;5(1):33-38.
Citicoline Protects Against Cognitive Impairment in a Rat Model of Chronic Cerebral Hypoperfusion
- Affiliations
-
- 1Department of Neurology, College of Medicine, The Catholic University of Korea, Seoul, Korea. nuyikim@catholic.ac.kr
Abstract
-
BACKGROUND AND PURPOSE: Cerebral white matter (WM) lesions are frequently observed in human cerebrovascular diseases, and are believed to be responsible for cognitive impairment. Various neuroprotective agents can suppress this type of WM or neuronal damage. In this study, we investigated whether citicoline, a drug used to treat acute ischemic stroke, can attenuate WM lesions and cognitive decline caused by chronic hypoperfusion in the rat.
METHODS
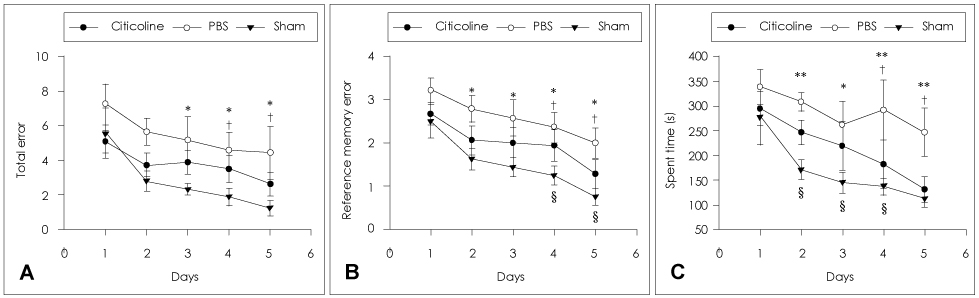
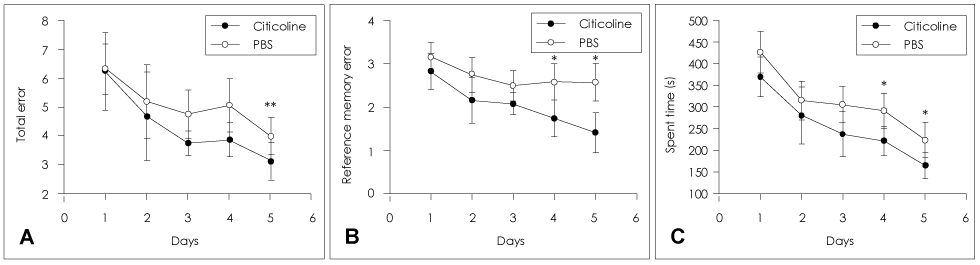
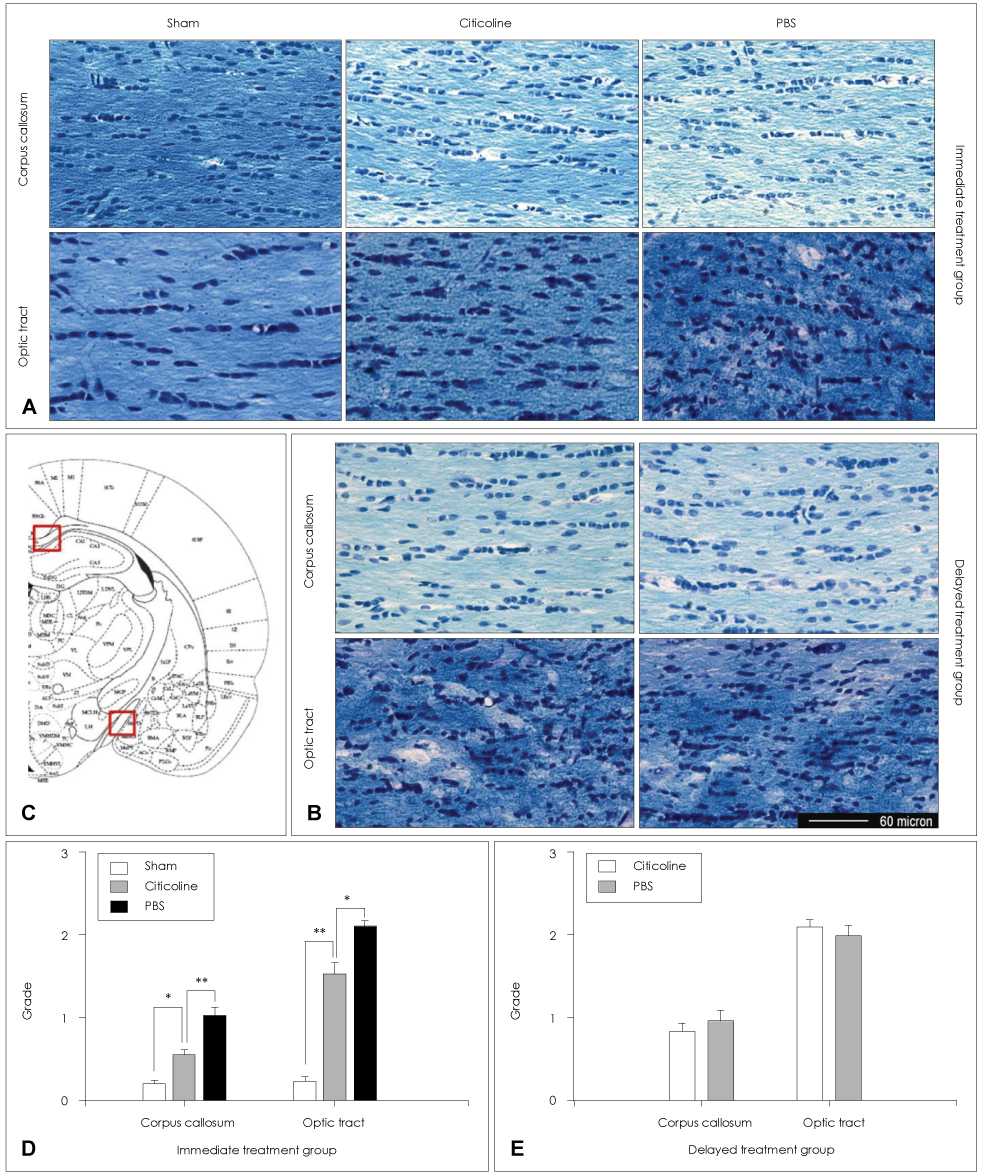
Animals were divided into immediate- and delayed-treatment groups. Those in the immediate-treatment group received a sham operation, citicoline (500 mg/kg/day), or phosphate buffered saline (PBS) treatment. Citicoline or PBS was administered intraperitoneally for 21 days after occluding the bilateral common carotid arteries. Rats in the delayed-treatment group were intraperitoneally administered with either 500 mg/kg/day citicoline or PBS for 21 days beginning on the 8th day after the operation. From the 17th day of administration, the rats were placed in an eight-arm radial maze to examine their cognitive abilities. After completing the administration, tissues were isolated for Kluver-Barrera and the terminal deoxynucleotidyl transferase biotin-dUTP nick end labelling (TUNEL) staining.
RESULTS
In the immediate-treatment group, cognitive functions were preserved in the citicoline-treated group, and WM damage and TUNEL-positive cells differed significantly between the citicoline- and PBS-treated animals. In the delayed-treatment group, there was no decrease in WM damage and TUNEL-positive cells, but cognitive improvement was evident for citicoline treatment relative to PBS treatment.
CONCLUSIONS
These results show that citicoline can prevent WM damage and aid cognitive improvement, even after a certain extent of disease progression. Citicoline might be useful in patients with acute ischemic stroke as well as in chronic stroke accompanied with cognitive impairment.
MeSH Terms
Figure
Reference
-
1. Sachdev PS, Brodaty H, Looi JC. Vascular dementia: diagnosis, management and possible prevention. Med J Aust. 1999. 170:81–85.
Article2. Farkas E, Donka G, de Vos RA, Mihály A, Bari F, Luiten PG. Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol. 2004. 108:57–64.
Article3. Stenset V, Hofoss D, Berstad AE, Negaard A, Gjerstad L, Fladby T. White matter lesion subtypes and cognitive deficits in patients with memory impairment. Dement Geriatr Cogn Disord. 2008. 26:424–431.
Article4. Fisher CM. Binswanger's encephalopathy: a review. J Neurol. 1989. 236:65–79.
Article5. Wakita H, Tomimoto H, Akiguchi I, Kimura J. Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: an immunohistochemical study. Acta Neuropathol. 1994. 87:484–492.
Article6. Kim JE, Lee BR, Chun JE, Lee SJ, Lee BH, Yu IK, et al. Cognitive Dysfunction in 16 patients with carotid stenosis: detailed neuropsychological Findings. J Clin Neurol. 2007. 3:9–17.
Article7. Kumaran D, Udayabanu M, Kumar M, Aneja R, Katyal A. Involvement of angiotensin converting enzyme in cerebral hypoperfusion induced anterograde memory impairment and cholinergic dysfunction in rats. Neuroscience. 2008. 155:626–639.
Article8. Otori T, Katsumata T, Katayama Y, Terashi A. Measurement of regional cerebral blood flow and glucose utilization in rat brain under chronic hypoperfusion conditions following bilateral carotid artery occlusion. Analyzed by autoradiographical methods. Nippon Ika Daigaku Zasshi. 1997. 64:428–439.
Article9. Tomimoto H, Akiguchi I, Wakita H, Kimura J. White matter lesions after occlusion of the bilateral carotid arteries in the rat--temporal profile of cerebral blood flow (CBF), oligodendroglia and myelin. No To Shinkei. 1997. 49:639–644.10. Tsuchiya M, Sako K, Yura S, Yonemasu Y. Cerebral blood flow and histopathological changes following permanent bilateral carotid artery ligation in Wistar rats. Exp Brain Res. 1992. 89:87–92.
Article11. Adibhatla RM, Hatcher JF. Citicoline mechanisms and clinical efficacy in cerebral ischemia. J Neurosci Res. 2002. 70:133–139.
Article12. Adibhatla RM, Hatcher JF, Dempsey RJ. Citicoline: neuroprotective mechanisms in cerebral ischemia. J Neurochem. 2002. 80:12–23.
Article13. Hurtado O, Moro MA, Cárdenas A, Sánchez V, Fernández-Tomé P, Leza JC, et al. Neuroprotection afforded by prior citicoline administration in experimental brain ischemia: effects on glutamate transport. Neurobiol Dis. 2005. 18:336–345.
Article14. Davidson CM, Pappas BA, Stevens WD, Fortin T, Bennett SA. Chronic cerebral hypoperfusion: loss of pupillary reflex, visual impairment and retinal neurodegeneration. Brain Res. 2000. 859:96–103.
Article15. Paxinos G, Watson C. The rat brain in stereotaxic coodinates. 1998. 4th ed. San Diego: Academic press.16. Wakita H, Tomimoto H, Akiguchi I, Kimura J. Dose-dependent, protective effect of FK506 against white matter changes in the rat brain after chronic cerebral ischemia. Brain Res. 1998. 792:105–113.
Article17. Tomimoto H, Ihara M, Wakita H, Ohtani R, Lin JX, Akiguchi I, et al. Chronic cerebral hypoperfusion induces white matter lesions and loss of oligodendroglia with DNA fragmentation in the rat. Acta Neuropathol. 2003. 106:527–534.
Article18. Parnetti L, Mignini F, Tomassoni D, Traini E, Amenta F. Cholinergic precursors in the treatment of cognitive impairment of vascular origin: ineffective approaches or need for re-evaluation? J Neurol Sci. 2007. 257:264–269.
Article19. Onal MZ, Li F, Tatlisumak T, Locke KW, Sandage BW Jr, Fisher M. Synergistic effects of citicoline and MK-801 in temporary experimental focal ischemia in rats. Stroke. 1997. 28:1060–1065.
Article20. Paul RH, Cohen RA, Moser DJ, Ott BR, Sethi M, Sweet L, et al. Clinical correlates of cognitive decline in vascular dementia. Cogn Behav Neurol. 2003. 16:40–46.
Article21. Agut J, Lopez G-Coviella I, Ortiz JA, Wurtman RJ. Oral cytidine 5'-diphosphate choline administration to rats increases brain phospholipid levels. Ann N Y Acad Sci. 1993. 695:318–320.
Article22. Secades JJ, Lorenzo JL. Citicoline: pharmacological and clinical review, 2006 update. Methods Find Exp Clin Pharmacol. 2006. 28:Suppl B. 1–56.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ERRATUM: Citicoline Protects Against Cognitive Impairment in a Rat Model of Chronic Cerebral Hypoperfusion
- Protective Effects of Citicoline and Benfotiamine Each Alone and in Combination on Streptozotocin-induced Memory Impairment in Mice
- Magnesium Increases the Protective Effect of Citicoline on Aluminum Chloride-induced Cognitive Impairment
- Preischemic Treadmill Exercise Ameliorates Memory Impairment and Microvasculature Damage in Rat Model of Chronic Cerebral Hypoperfusion
- Neuroprotective Effect of Duloxetine on Chronic Cerebral Hypoperfusion-Induced Hippocampal Neuronal Damage