Cancer Res Treat.
2017 Oct;49(4):915-926. 10.4143/crt.2016.322.
Patient-Derived Xenograft Models of Epithelial Ovarian Cancer for Preclinical Studies
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 2Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. garden.lee@samsung.com
- 3Department of Clinical Oncology, Queen Elizabeth Hospital, Kowloon, Hong Kong.
- 4Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Institute for Refractory Cancer Research, Samsung Medical Center, Seoul, Korea.
- 6Division of Gynecologic Oncology, Princess Margaret Cancer Centre, University Health Network, University of Toronto, Toronto, Canada.
- 7Samsung Genome Institute, Samsung Medical Center, Seoul, Korea.
- 8Samsung Advanced Institute for Health Sciences and Technology, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2394811
- DOI: http://doi.org/10.4143/crt.2016.322
Abstract
- PURPOSE
Patient-derived tumor xenografts (PDXs) can provide more reliable information about tumor biology than cell line models. We developed PDXs for epithelial ovarian cancer (EOC) that have histopathologic and genetic similarities to the primary patient tissues and evaluated their potential for use as a platform for translational EOC research.
MATERIALS AND METHODS
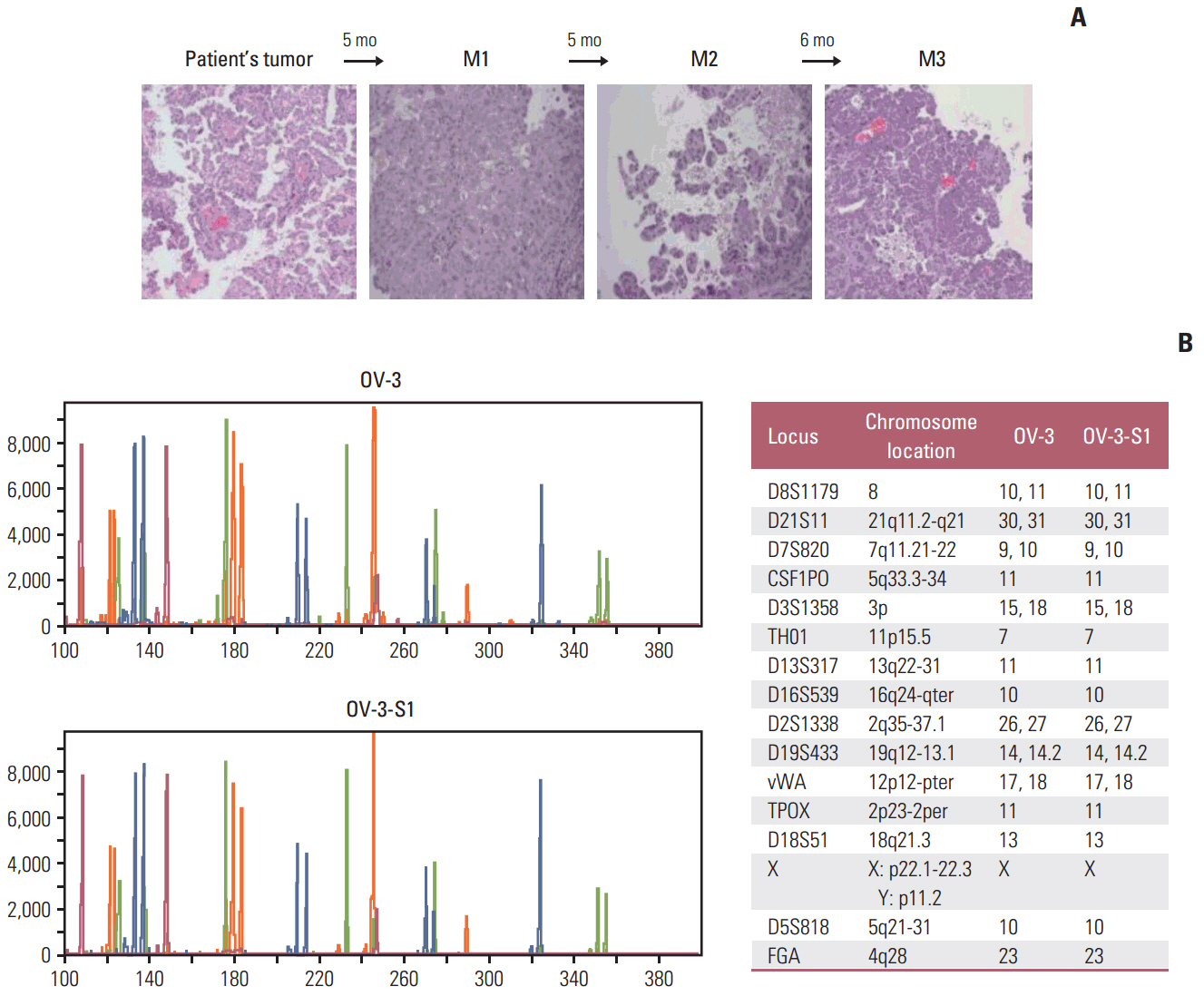
We successfully established PDXs by subrenal capsule implantation of primary EOC tissues into female BALB/C-nude mice. The rate of successful PDX engraftment was 48.8% (22/45 cases). Hematoxylin and eosin staining and short tandem repeat analysis showed histopathological and genetic similarity between the PDX and primary patient tissues.
RESULTS
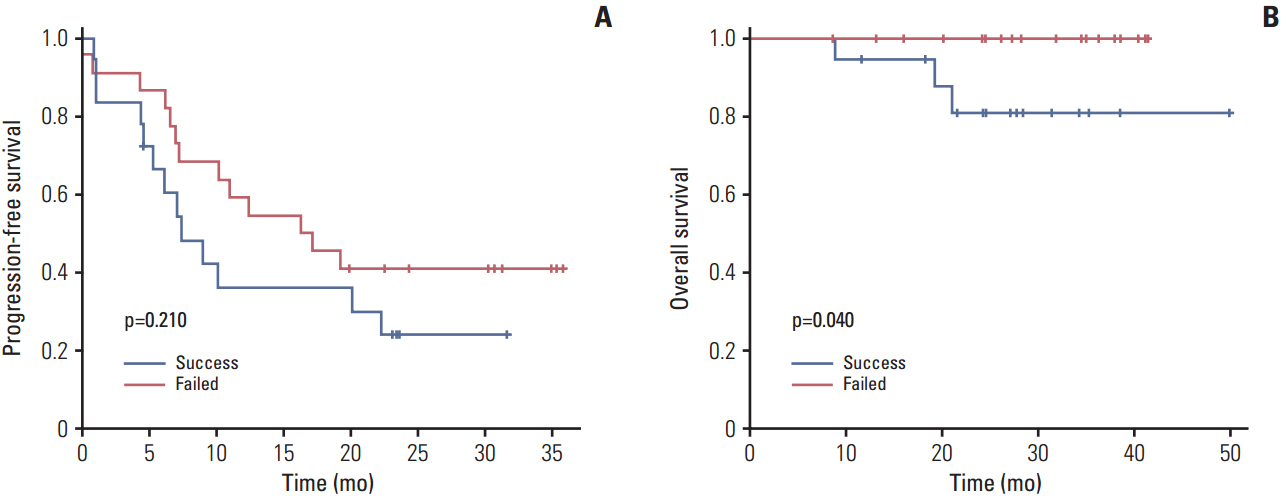
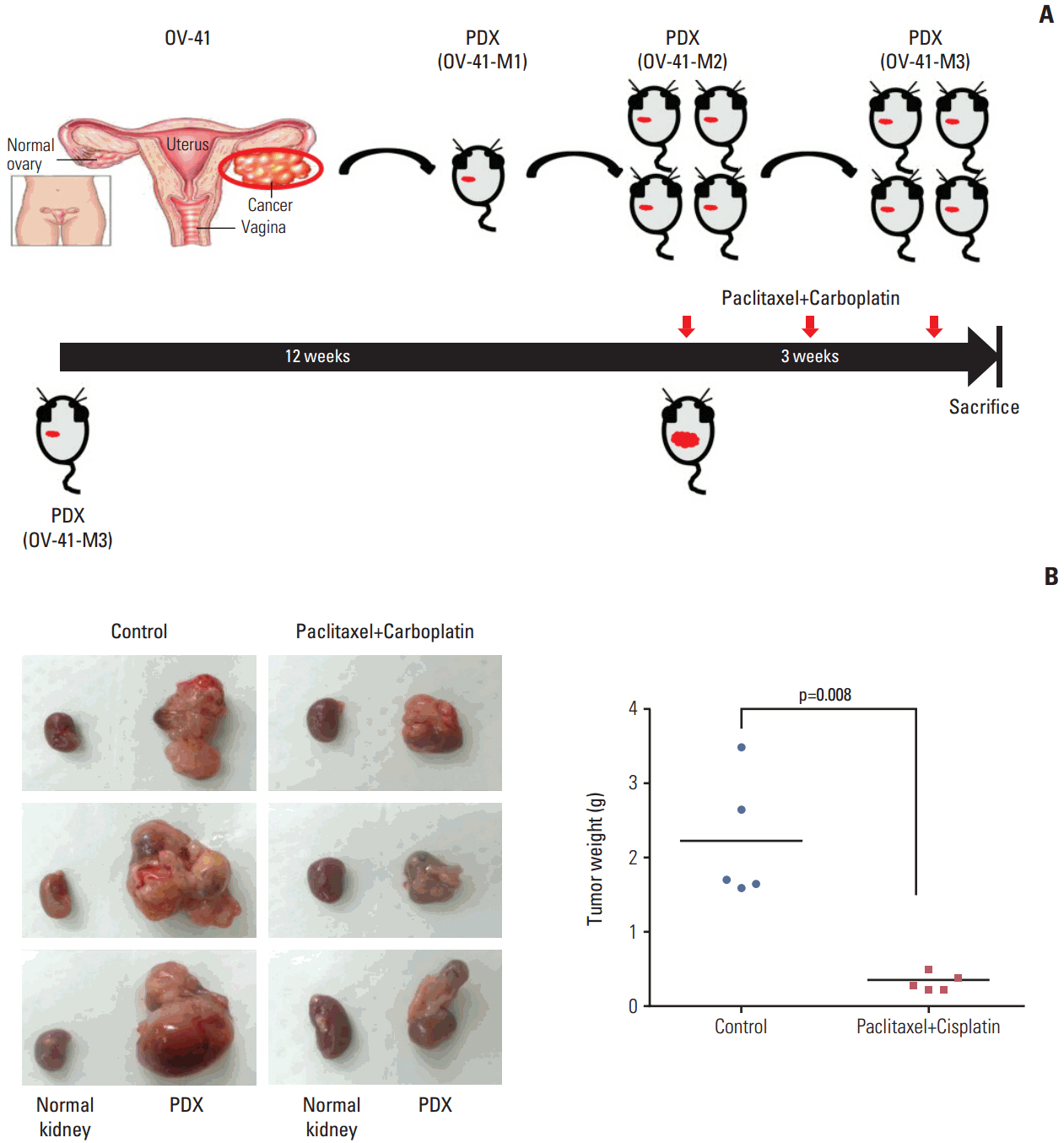
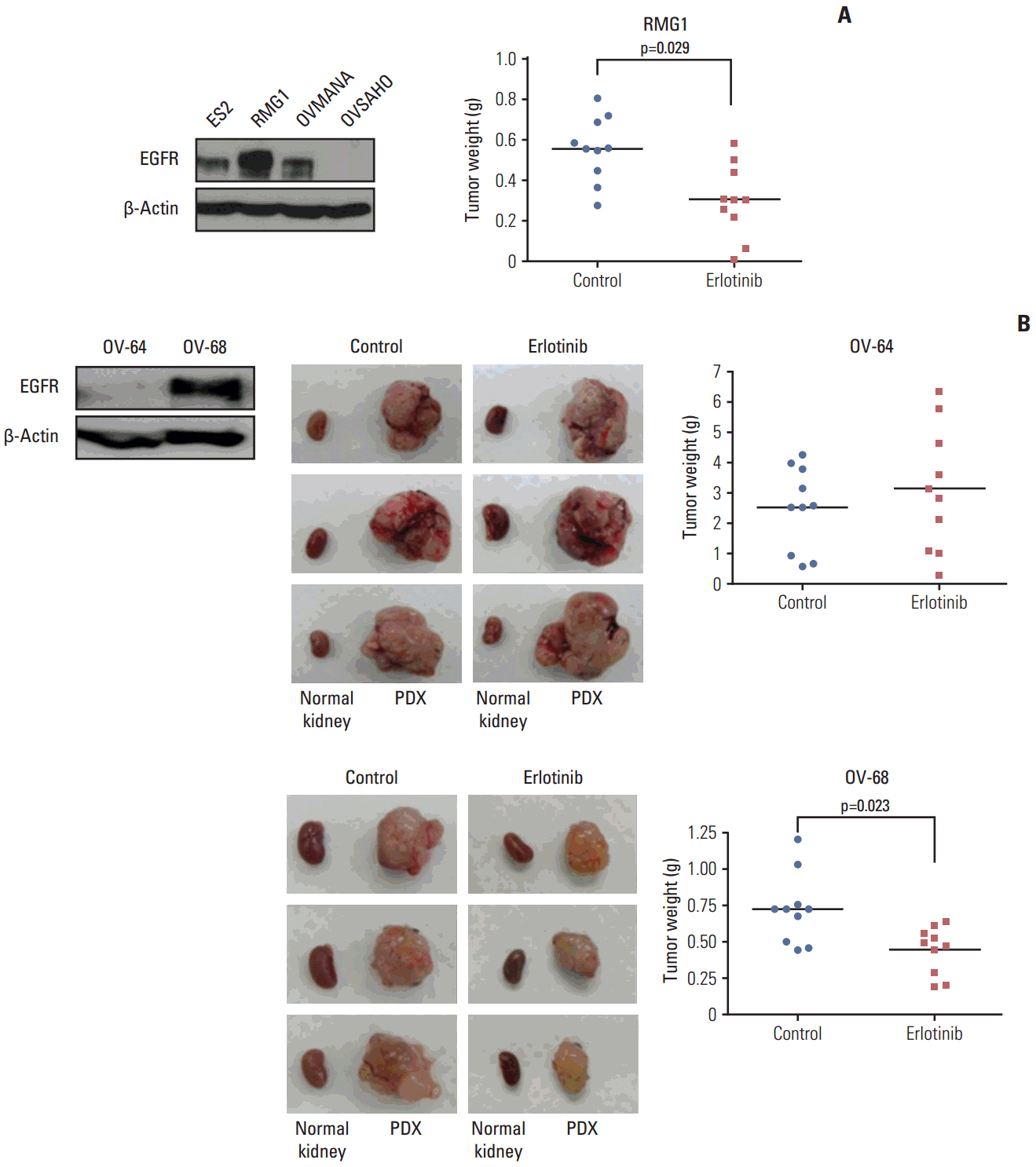
Patients whose tumors were successfully engrafted in mice had significantly inferior overall survival when compared with those whose tumors failed to engraft (p=0.040). In preclinical tests of this model, we found that paclitaxel-carboplatin combination chemotherapy significantly deceased tumor weight in PDXs compared with the control treatment (p=0.013). Moreover, erlotinib treatment significantly decreased tumor weight in epidermal growth factor receptor-overexpressing PDX with clear cell histology (p=0.023).
CONCLUSION
PDXs for EOC with histopathological and genetic stability can be efficiently developed by subrenal capsule implantation and have the potential to provide a promising platform for future translational research and precision medicine for EOC.
Keyword
MeSH Terms
-
Animals
Biology
Cell Line
Drug Therapy, Combination
Eosine Yellowish-(YS)
Epidermal Growth Factor
Erlotinib Hydrochloride
Female
Hematoxylin
Heterografts*
Humans
Mice
Microsatellite Repeats
Molecular Targeted Therapy
Ovarian Neoplasms*
Precision Medicine
Translational Medical Research
Tumor Burden
Eosine Yellowish-(YS)
Epidermal Growth Factor
Erlotinib Hydrochloride
Hematoxylin
Figure
Cited by 1 articles
-
Comparison of Clinical Features and Outcomes in Epithelial Ovarian Cancer according to Tumorigenicity in Patient-Derived Xenograft Models
Kyung Jin Eoh, Young Shin Chung, So Hyun Lee, Sun-Ae Park, Hee Jung Kim, Wookyeom Yang, In Ok Lee, Jung-Yun Lee, Hanbyoul Cho, Doo Byung Chay, Sunghoon Kim, Sang Wun Kim, Jae-Hoon Kim, Young Tae Kim, Eun Ji Nam
Cancer Res Treat. 2018;50(3):956-963. doi: 10.4143/crt.2017.181.
Reference
-
References
1. Duffy MJ. The war on cancer: are we winning? Tumour Biol. 2013; 34:1275–84.
Article2. Scott CL, Becker MA, Haluska P, Samimi G. Patient-derived xenograft models to improve targeted therapy in epithelial ovarian cancer treatment. Front Oncol. 2013; 3:295.
Article3. Stordal B, Timms K, Farrelly A, Gallagher D, Busschots S, Renaud M, et al. BRCA1/2 mutation analysis in 41 ovarian cell lines reveals only one functionally deleterious BRCA1 mutation. Mol Oncol. 2013; 7:567–79.
Article4. Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012; 9:338–50.
Article5. Boone JD, Dobbin ZC, Straughn JM Jr, Buchsbaum DJ. Ovarian and cervical cancer patient derived xenografts: the past, present, and future. Gynecol Oncol. 2015; 138:486–91.
Article6. Lee CH, Xue H, Sutcliffe M, Gout PW, Huntsman DG, Miller DM, et al. Establishment of subrenal capsule xenografts of primary human ovarian tumors in SCID mice: potential models. Gynecol Oncol. 2005; 96:48–55.
Article7. Press JZ, Kenyon JA, Xue H, Miller MA, De Luca A, Miller DM, et al. Xenografts of primary human gynecological tumors grown under the renal capsule of NOD/SCID mice show genetic stability during serial transplantation and respond to cytotoxic chemotherapy. Gynecol Oncol. 2008; 110:256–64.
Article8. Watanabe H, Okada M, Kaji Y, Satouchi M, Sato Y, Yamabe Y, et al. New response evaluation criteria in solid tumoursrevised RECIST guideline (version 1.1). Gan To Kagaku Ryoho. 2009; 36:2495–501.9. Oh DY, Kim S, Choi YL, Cho YJ, Oh E, Choi JJ, et al. HER2 as a novel therapeutic target for cervical cancer. Oncotarget. 2015; 6:36219–30.
Article10. Staflin K, Jarnum S, Hua J, Honeth G, Kannisto P, Lindvall M. Combretastatin A-1 phosphate potentiates the antitumor activity of carboplatin and paclitaxel in a severe combined immunodeficiency disease (SCID) mouse model of human ovarian carcinoma. Int J Gynecol Cancer. 2006; 16:1557–64.
Article11. Sangodkar J, Dhawan NS, Melville H, Singh VJ, Yuan E, Rana H, et al. Targeting the FOXO1/KLF6 axis regulates EGFR signaling and treatment response. J Clin Invest. 2012; 122:2637–51.
Article12. Sereni MI, Baldelli E, Gambara G, Deng J, Zanotti L, Bandiera E, et al. Functional characterization of epithelial ovarian cancer histotypes by drug target based protein signaling activation mapping: implications for personalized cancer therapy. Proteomics. 2015; 15:365–73.
Article13. Dobbin ZC, Katre AA, Steg AD, Erickson BK, Shah MM, Alvarez RD, et al. Using heterogeneity of the patient-derived xenograft model to identify the chemoresistant population in ovarian cancer. Oncotarget. 2014; 5:8750–64.
Article14. Elkas JC, Baldwin RL, Pegram M, Tseng Y, Slamon D, Karlan BY. A human ovarian carcinoma murine xenograft model useful for preclinical trials. Gynecol Oncol. 2002; 87:200–6.
Article15. Ricci F, Bizzaro F, Cesca M, Guffanti F, Ganzinelli M, Decio A, et al. Patient-derived ovarian tumor xenografts recapitulate human clinicopathology and genetic alterations. Cancer Res. 2014; 74:6980–90.
Article16. Topp MD, Hartley L, Cook M, Heong V, Boehm E, McShane L, et al. Molecular correlates of platinum response in human high-grade serous ovarian cancer patient-derived xenografts. Mol Oncol. 2014; 8:656–68.
Article17. Weroha SJ, Becker MA, Enderica-Gonzalez S, Harrington SC, Oberg AL, Maurer MJ, et al. Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clin Cancer Res. 2014; 20:1288–97.
Article18. Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014; 4:998–1013.
Article19. Zhu G, Hong L, Wang S, Junhong C, Yang Z, Yao M. Comparison of two kinds of orthotopic xenograft models for human ovarian cancer. Eur J Gynaecol Oncol. 2014; 35:724–7.20. Jin K, Teng L, Shen Y, He K, Xu Z, Li G. Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clin Transl Oncol. 2010; 12:473–80.
Article21. Reyal F, Guyader C, Decraene C, Lucchesi C, Auger N, Assayag F, et al. Molecular profiling of patient-derived breast cancer xenografts. Breast Cancer Res. 2012; 14:R11.
Article22. Vergote IB, Jimeno A, Joly F, Katsaros D, Coens C, Despierre E, et al. Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy for ovarian carcinoma: a European Organisation for Research and Treatment of Cancer-Gynaecological Cancer Group, and Gynecologic Cancer Intergroup study. J Clin Oncol. 2014; 32:320–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Generation and Application of Patient-Derived Xenograft Model for Cancer Research
- Sex-dependent liver cancer xenograft models for predicting clinical data in the evaluation of anticancer drugs
- Patient-derived xenografts as compatible models for precision oncology
- Preclinical Models of Follicular Cell-Derived Thyroid Cancer: An Overview from Cancer Cell Lines to Mouse Models
- Anti-cancer effect of fenbendazole-incorporated PLGA nanoparticles in ovarian cancer