Diabetes Metab J.
2017 Aug;41(4):303-315. 10.4093/dmj.2017.41.4.303.
In Vitro Effect of Fatty Acids Identified in the Plasma of Obese Adolescents on the Function of Pancreatic β-Cells
- Affiliations
-
- 1Research Group in Food and Human Nutrition, School of Dietetics and Human Nutrition, University of Antioquia, Medellin, Colombia.
- 2Genetics Molecular Group, University of Antioquia, Medellin, Colombia. norman.balcazar@udea.edu.co
- 3Department of Physiology and Biochemistry, School of Medicine, University of Antioquia, Medellin, Colombia.
- KMID: 2392471
- DOI: http://doi.org/10.4093/dmj.2017.41.4.303
Abstract
- BACKGROUND
The increase in circulating free fatty acid (FFA) levels is a major factor that induces malfunction in pancreatic β-cells. We evaluated the effect of FFAs reconstituted according to the profile of circulating fatty acids found in obese adolescents on the viability and function of the murine insulinoma cell line (mouse insulinoma [MIN6]).
METHODS
From fatty acids obtained commercially, plasma-FFA profiles of three different youth populations were reconstituted: obese with metabolic syndrome; obese without metabolic syndrome; and normal weight without metabolic syndrome. MIN6 cells were treated for 24 or 48 hours with the three FFA profiles, and glucose-stimulated insulin secretion, cell viability, mitochondrial function and antioxidant activity were evaluated.
RESULTS
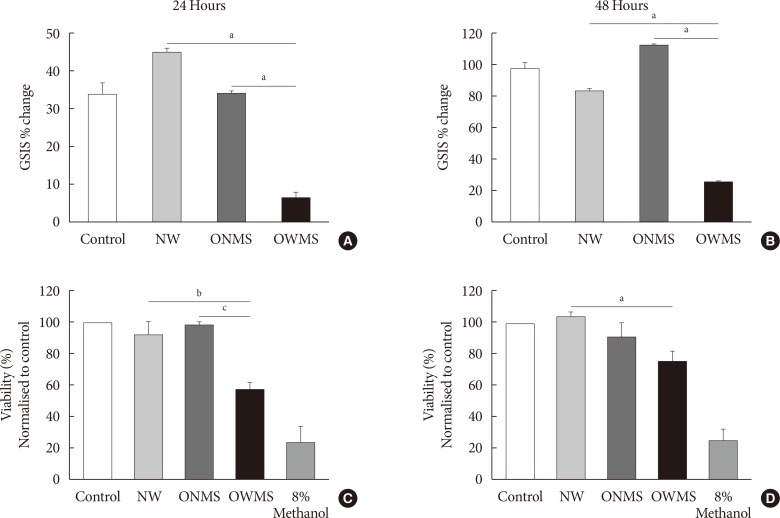
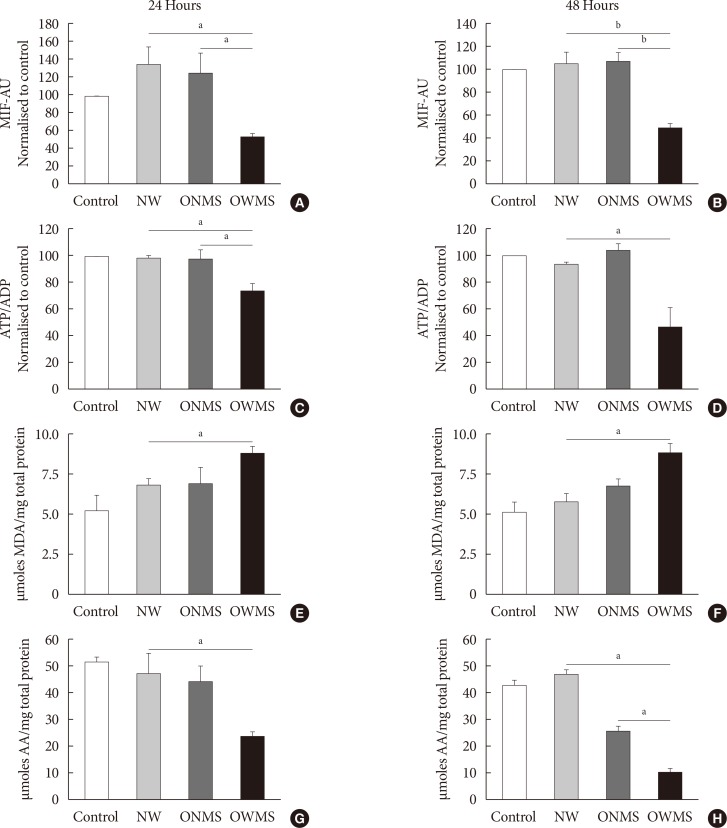
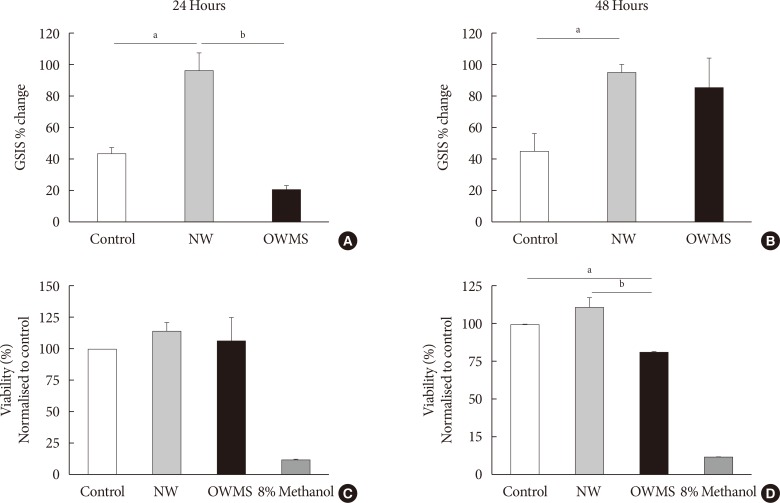
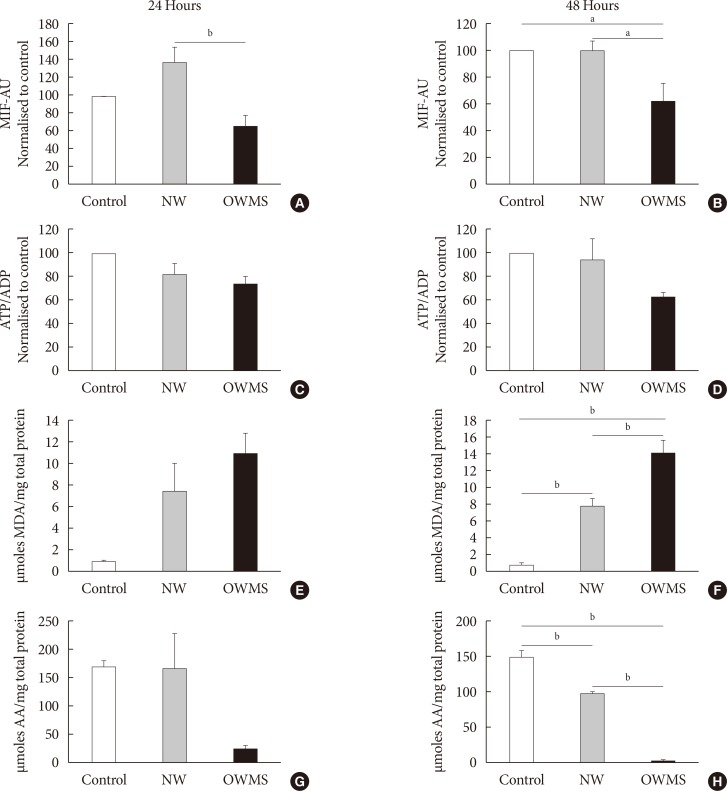
The high FFA content and high polyunsaturated ω6/ω3 ratio, present in plasma of obese adolescents with metabolic syndrome had a toxic effect on MIN6 cell viability and function, increasing oxidative stress and decreasing glucose-dependent insulin secretion.
CONCLUSION
These results could help to guide nutritional management of obese young individuals, encouraging the increase of ω-3-rich food consumption in order to reduce the likelihood of deterioration of β-cells and the possible development of type 2 diabetes mellitus.
Keyword
MeSH Terms
Figure
Reference
-
1. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 192 million participants. Lancet. 2016; 387:1377–1396. PMID: 27115820.2. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999; 282:1523–1529. PMID: 10546691.
Article3. Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003; 289:76–79. PMID: 12503980.
Article4. Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006; 444:881–887. PMID: 17167477.
Article5. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444:860–867. PMID: 17167474.
Article6. Kloting N, Bluher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord. 2014; 15:277–287. PMID: 25344447.
Article7. Furstova V, Kopska T, James RF, Kovar J. Comparison of the effect of individual saturated and unsaturated fatty acids on cell growth and death induction in the human pancreatic beta-cell line NES2Y. Life Sci. 2008; 82:684–691. PMID: 18272185.8. Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology. 2006; 147:3398–3407. PMID: 16601139.9. Lai E, Bikopoulos G, Wheeler MB, Rozakis-Adcock M, Volchuk A. Differential activation of ER stress and apoptosis in response to chronically elevated free fatty acids in pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2008; 294:E540–E550. PMID: 18198352.10. Bermudez JA, Velasquez CM. Profile of free fatty acids (FFA) in serum of young Colombians with obesity and metabolic syndrome. Arch Latinoam Nutr. 2014; 64:248–257. PMID: 26336720.11. Berridge MV, Tan AS. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys. 1993; 303:474–482. PMID: 8390225.
Article12. Rojano BA, Gaviria CA, Saez JA. Antioxidant activity determination in a lipidic peroxidation model of butter inhibited by isoespintanol. Vitae. 2008; 15:212–218.13. Whiteman M, Ketsawatsakul U, Halliwell B. A reassessment of the peroxynitrite scavenging activity of uric acid. Ann N Y Acad Sci. 2002; 962:242–259. PMID: 12076979.
Article14. Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 2001; 50:69–76. PMID: 11147797.15. Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003; 52:726–733. PMID: 12606514.16. Kawashima A, Sugawara S, Okita M, Akahane T, Fukui K, Hashiuchi M, Kataoka C, Tsukamoto I. Plasma fatty acid composition, estimated desaturase activities, and intakes of energy and nutrient in Japanese men with abdominal obesity or metabolic syndrome. J Nutr Sci Vitaminol (Tokyo). 2009; 55:400–406. PMID: 19926925.
Article17. Klein-Platat C, Drai J, Oujaa M, Schlienger JL, Simon C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am J Clin Nutr. 2005; 82:1178–1184. PMID: 16332649.
Article18. Malmgren S, Spegel P, Danielsson AP, Nagorny CL, Andersson LE, Nitert MD, Ridderstrale M, Mulder H, Ling C. Coordinate changes in histone modifications, mRNA levels, and metabolite profiles in clonal INS-1 832/13 beta-cells accompany functional adaptations to lipotoxicity. J Biol Chem. 2013; 288:11973–11987. PMID: 23476019.19. Akash MS, Rehman K, Chen S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2013; 114:525–531. PMID: 22991242.
Article20. Haber EP, Hirabara SM, Gomes AD, Curi R, Carpinelli AR, Carvalho CR. Palmitate modulates the early steps of insulin signalling pathway in pancreatic islets. FEBS Lett. 2003; 544:185–188. PMID: 12782313.
Article21. Pi J, Collins S. Reactive oxygen species and uncoupling protein 2 in pancreatic beta-cell function. Diabetes Obes Metab. 2010; 12(Suppl 2):141–148. PMID: 21029311.22. Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012; 18:59–68. PMID: 21889406.
Article23. Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005; 307:384–387. PMID: 15662004.
Article24. Luo P, Wang MH. Eicosanoids, beta-cell function, and diabetes. Prostaglandins Other Lipid Mediat. 2011; 95:1–10. PMID: 21757024.25. Meng ZX, Sun JX, Ling JJ, Lv JH, Zhu DY, Chen Q, Sun YJ, Han X. Prostaglandin E2 regulates Foxo activity via the Akt pathway: implications for pancreatic islet beta cell dysfunction. Diabetologia. 2006; 49:2959–2968. PMID: 17033838.
Article26. Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M, Bergamini E, Mosca F, Di Mario U, Del Prato S, Marchetti P. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes. 2002; 51:1437–1442. PMID: 11978640.27. Eitel K, Staiger H, Rieger J, Mischak H, Brandhorst H, Brendel MD, Bretzel RG, Haring HU, Kellerer M. Protein kinase C delta activation and translocation to the nucleus are required for fatty acid-induced apoptosis of insulin-secreting cells. Diabetes. 2003; 52:991–997. PMID: 12663471.28. Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ. Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic beta-cells (INS-1). J Biol Chem. 2002; 277:49676–49684. PMID: 12393870.29. Rakatzi I, Mueller H, Ritzeler O, Tennagels N, Eckel J. Adiponectin counteracts cytokine- and fatty acid-induced apoptosis in the pancreatic beta-cell line INS-1. Diabetologia. 2004; 47:249–258. PMID: 14722646.
Article30. Mehmeti I, Gurgul-Convey E, Lenzen S, Lortz S. Induction of the intrinsic apoptosis pathway in insulin-secreting cells is dependent on oxidative damage of mitochondria but independent of caspase-12 activation. Biochim Biophys Acta. 2011; 1813:1827–1835. PMID: 21784110.
Article31. Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007; 583(Pt 1):9–24. PMID: 17584843.
Article32. Lim S, Rashid MA, Jang M, Kim Y, Won H, Lee J, Woo JT, Kim YS, Murphy MP, Ali L, Ha J, Kim SS. Mitochondria-targeted antioxidants protect pancreatic beta-cells against oxidative stress and improve insulin secretion in glucotoxicity and glucolipotoxicity. Cell Physiol Biochem. 2011; 28:873–886. PMID: 22178940.33. Lin N, Chen H, Zhang H, Wan X, Su Q. Mitochondrial reactive oxygen species (ROS) inhibition ameliorates palmitate-induced INS-1 beta cell death. Endocrine. 2012; 42:107–117. PMID: 22350662.
Article34. Wei D, Li J, Shen M, Jia W, Chen N, Chen T, Su D, Tian H, Zheng S, Dai Y, Zhao A. Cellular production of n-3 PUFAs and reduction of n-6-to-n-3 ratios in the pancreatic beta-cells and islets enhance insulin secretion and confer protection against cytokine-induced cell death. Diabetes. 2010; 59:471–478. PMID: 19933995.35. Simopoulos AP. Importance of the ratio of omega-6/omega-3 essential fatty acids: evolutionary aspects. World Rev Nutr Diet. 2003; 92:1–22. PMID: 14579680.
Article36. Calder PC. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006; 83(6 Suppl):1505S–1519S. PMID: 16841861.
Article37. Linn T, Noke M, Woehrle M, Kloer HU, Hammes HP, Litzlbauer D, Bretzel RG, Federlin K. Fish oil-enriched diet and reduction of low-dose streptozocin-induced hyperglycemia. Inhibition of macrophage activation. Diabetes. 1989; 38:1402–1411. PMID: 2533572.
Article38. Gonzalez-Periz A, Horrillo R, Ferre N, Gronert K, Dong B, Moran-Salvador E, Titos E, Martinez-Clemente M, Lopez-Parra M, Arroyo V, Claria J. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009; 23:1946–1957. PMID: 19211925.39. Kang JX. The importance of omega-6/omega-3 fatty acid ratio in cell function. The gene transfer of omega-3 fatty acid desaturase. World Rev Nutr Diet. 2003; 92:23–36. PMID: 14579681.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypertriglyceridemia in Obese Children and Adolescents
- Differential Effects of Palmitate and Docosahexaenoic acid on ATP-sensitive K+ Channel Activity of Pancreatic beta-cells
- Anti-cancer Mechanism of Docosahexaenoic Acid in Pancreatic Carcinogenesis: A Mini-review
- Lowering of plasma free fatty acids by acipimox increases insulin sensitivity in rats
- An Update on Hypertriglyceridemia-Induced Acute Pancreatitis