J Bone Metab.
2014 Feb;21(1):29-40.
Interaction between Muscle and Bone
- Affiliations
-
- 1Department of Physiology and Regenerative Medicine, Kinki University Faculty of Medicine, Osaka, Japan. hkaji@med.kindai.ac.jp
Abstract
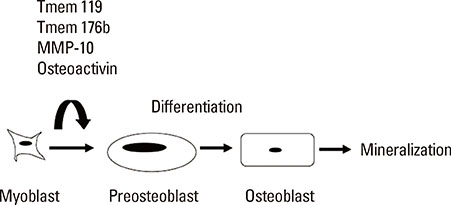
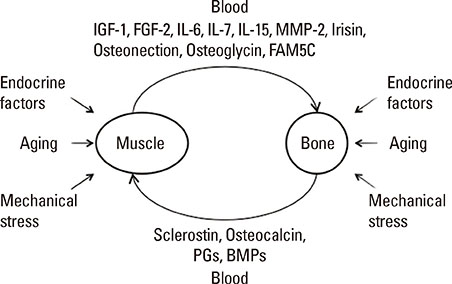
- The clinical significance of sarcopenia and osteoporosis has increased with the increase in the population of older people. Sarcopenia is defined by decreased muscle mass and impaired muscle function, which is related to osteoporosis independently and dependently. Numerous lines of clinical evidence suggest that lean body mass is positively related to bone mass, which leads to reduced fracture risk. Genetic, endocrine and mechanical factors affect both muscle and bone simultaneously. Vitamin D, the growth hormone/insulin-like growth factor I axis and testosterone are physiologically and pathologically important as endocrine factors. These findings suggest the presence of interactions between muscle and bone, which might be very important for understanding the physiology and pathophysiology of sarcopenia and osteoporosis. Muscle/bone relationships include two factors: local control of muscle to bone and systemic humoral interactions between muscle and bone. As a putative local inducer of muscle ossification, we found Tmem119, a parathyroid hormone-responsive osteoblast differentiation factor. Moreover, osteoglycin might be one of the muscle-derived humoral bone anabolic factors. This issue may be important for the development of novel drugs and biomarkers for osteoporosis and sarcopenia. Further research will be necessary to clarify the details of the linkage of muscle and bone.
Keyword
MeSH Terms
Figure
Reference
-
1. Miyakoshi N, Hongo M, Mizutani Y, et al. Prevalence of sarcopenia in Japanese women with osteopenia and osteoporosis. J Bone Miner Metab. 2013; 31:556–561.
Article2. Bonewald LF, Kiel DP, Clemens TL, et al. Forum on bone and skeletal muscle interactions: summary of the proceedings of an ASBMR workshop. J Bone Miner Res. 2013; 28:1857–1865.
Article3. Ong T, Sahota O, Tan W, et al. A United Kingdom perspective on the relationship between body mass index (BMI) and bone health: a cross sectional analysis of data from the Nottingham Fracture Liaison Service. Bone. 2014; 59:207–210.
Article4. Verschueren S, Gielen E, O'Neill TW, et al. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporos Int. 2013; 24:87–98.
Article5. Ducher G, Bass SL, Saxon L, et al. Effects of repetitive loading on the growth-induced changes in bone mass and cortical bone geometry: a 12-month study in pre/peri- and postmenarcheal tennis players. J Bone Miner Res. 2011; 26:1321–1329.
Article6. Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture in men and women: an epidemiologic perspective. J Bone Miner Res. 2012; 27:1–10.
Article7. Nielson CM, Marshall LM, Adams AL, et al. BMI and fracture risk in older men: the osteoporotic fractures in men study (MrOS). J Bone Miner Res. 2011; 26:496–502.
Article8. Johannesdottir F, Aspelund T, Siggeirsdottir K, et al. Mid-thigh cortical bone structural parameters, muscle mass and strength, and association with lower limb fractures in older men and women (AGES-Reykjavik Study). Calcif Tissue Int. 2012; 90:354–364.
Article9. Kaji H. Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care. 2013; 16:272–277.10. Cooper C, Dere W, Evans W, et al. Frailty and sarcopenia: definitions and outcome parameters. Osteoporos Int. 2012; 23:1839–1848.
Article11. Rikkonen T, Sirola J, Salovaara K, et al. Muscle strength and body composition are clinical indicators of osteoporosis. Calcif Tissue Int. 2012; 91:131–138.
Article12. Shah K, Armamento-Villareal R, Parimi N, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011; 26:2851–2859.
Article13. Armamento-Villareal R, Sadler C, Napoli N, et al. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res. 2012; 27:1215–1221.
Article14. Sornay-Rendu E, Karras-Guillibert C, Munoz F, et al. Age determines longitudinal changes in body composition better than menopausal and bone status: the OFELY study. J Bone Miner Res. 2012; 27:628–636.
Article15. Wey HE, Binkley TL, Beare TM, et al. Cross-sectional versus longitudinal associations of lean and fat mass with pQCT bone outcomes in children. J Clin Endocrinol Metab. 2011; 96:106–114.
Article16. Reyes ML, Hernández M, Holmgren LJ, et al. High-frequency, low-intensity vibrations increase bone mass and muscle strength in upper limbs, improving autonomy in disabled children. J Bone Miner Res. 2011; 26:1759–1766.
Article17. Szulc P, Blaizot S, Boutroy S, et al. Impaired bone microarchitecture at the distal radius in older men with low muscle mass and grip strength: the STRAMBO study. J Bone Miner Res. 2013; 28:169–178.
Article18. Sharir A, Stern T, Rot C, et al. Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development. 2011; 138:3247–3259.
Article19. Karasik D, Kiel DP. Genetics of the musculoskeletal system: a pleiotropic approach. J Bone Miner Res. 2008; 23:788–802.
Article20. Bogl LH, Latvala A, Kaprio J, et al. An investigation into the relationship between soft tissue body composition and bone mineral density in a young adult twin sample. J Bone Miner Res. 2011; 26:79–87.
Article21. Karasik D, Cohen-Zinder M. The genetic pleiotropy of musculoskeletal aging. Front Physiol. 2012; 3:303.
Article22. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96:1911–1930.
Article23. Autier P, Gandini S, Mullie P. A systematic review: influence of vitamin D supplementation on serum 25-hydroxyvitamin D concentration. J Clin Endocrinol Metab. 2012; 97:2606–2613.
Article24. Nurmi-Lüthje I, Sund R, Juntunen M, et al. Post-hip fracture use of prescribed calcium plus vitamin D or vitamin D supplements and antiosteoporotic drugs is associated with lower mortality: a nationwide study in Finland. J Bone Miner Res. 2011; 26:1845–1853.
Article25. Rejnmark L, Avenell A, Masud T, et al. Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. J Clin Endocrinol Metab. 2012; 97:2670–2681.
Article26. Glendenning P, Zhu K, Inderjeeth C, et al. Effects of three-monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women: a randomized controlled trial. J Bone Miner Res. 2012; 27:170–176.
Article27. Marantes I, Achenbach SJ, Atkinson EJ, et al. Is vitamin D a determinant of muscle mass and strength? J Bone Miner Res. 2011; 26:2860–2871.
Article28. Garcia LA, King KK, Ferrini MG, et al. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology. 2011; 152:2976–2986.
Article29. Goldspink G. Age-related loss of muscle mass and strength. J Aging Res. 2012; 2012:158279.
Article30. Terracciano C, Celi M, Lecce D, et al. Differential features of muscle fiber atrophy in osteoporosis and osteoarthritis. Osteoporos Int. 2013; 24:1095–1100.
Article31. Van Caenegem E, Wierckx K, Taes Y, et al. Bone mass, bone geometry, and body composition in female-to-male transsexual persons after long-term cross-sex hormonal therapy. J Clin Endocrinol Metab. 2012; 97:2503–2511.
Article32. Birzniece V, Meinhardt UJ, Gibney J, et al. Differential effects of raloxifene and estrogen on body composition in growth hormone-replaced hypopituitary women. J Clin Endocrinol Metab. 2012; 97:1005–1012.
Article33. Lebrasseur NK, Achenbach SJ, Melton LJ 3rd, et al. Skeletal muscle mass is associated with bone geometry and microstructure and serum insulin-like growth factor binding protein-2 levels in adult women and men. J Bone Miner Res. 2012; 27:2159–2169.
Article34. Lang TF. The bone-muscle relationship in men and women. J Osteoporos. 2011; 2011:702735.
Article35. Rariy CM, Ratcliffe SJ, Weinstein R, et al. Higher serum free testosterone concentration in older women is associated with greater bone mineral density, lean body mass, and total fat mass: the cardiovascular health study. J Clin Endocrinol Metab. 2011; 96:989–996.
Article36. Kaji H, Tobimatsu T, Naito J, et al. Body composition and vertebral fracture risk in female patients treated with glucocorticoid. Osteoporos Int. 2006; 17:627–633.
Article37. Skversky AL, Kumar J, Abramowitz MK, et al. Association of glucocorticoid use and low 25-hydroxyvitamin D levels: results from the National Health and Nutrition Examination Survey (NHANES): 2001-2006. J Clin Endocrinol Metab. 2011; 96:3838–3845.
Article38. Butner KL, Creamer KW, Nickols-Richardson SM, et al. Fat and muscle indices assessed by pQCT: relationships with physical activity and type 2 diabetes risk. J Clin Densitom. 2012; 15:355–361.
Article39. Schwartz AV, Johnson KC, Kahn SE, et al. Effect of 1 year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: results from the Look AHEAD randomized trial. J Bone Miner Res. 2012; 27:619–627.
Article40. Wood RJ, O'Neill EC. Resistance training in type II diabetes mellitus: impact on areas of metabolic dysfunction in skeletal muscle and potential impact on bone. J Nutr Metab. 2012; 2012:268197.
Article41. Keyak JH, Koyama AK, LeBlanc A, et al. Reduction in proximal femoral strength due to long-duration spaceflight. Bone. 2009; 44:449–453.
Article42. Colnot C, Zhang X, Knothe Tate ML. Current insights on the regenerative potential of the periosteum: molecular, cellular, and endogenous engineering approaches. J Orthop Res. 2012; 30:1869–1878.
Article43. Evans SF, Parent JB, Lasko CE, et al. Periosteum, bone's "smart" bounding membrane, exhibits direction-dependent permeability. J Bone Miner Res. 2013; 28:608–617.
Article44. Henrotin Y. Muscle: a source of progenitor cells for bone fracture healing. BMC Med. 2011; 9:136.
Article45. Glass GE, Chan JK, Freidin A, et al. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011; 108:1585–1590.
Article46. Hisa I, Kawara A, Katagiri T, et al. Effects of serum from a fibrodysplasia ossificans progressiva patient on osteoblastic cells. Open J Endocr Metab Dis. 2012; 2:1–6.
Article47. Whyte MP, Wenkert D, Demertzis JL, et al. Fibrodysplasia ossificans progressiva: middle-age onset of heterotopic ossification from a unique missense mutation (c.974G>C, p.G325A) in ACVR1. J Bone Miner Res. 2012; 27:729–737.
Article48. Leblanc E, Trensz F, Haroun S, et al. BMP-9-induced muscle heterotopic ossification requires changes to the skeletal muscle microenvironment. J Bone Miner Res. 2011; 26:1166–1177.
Article49. Shi S, de Gorter DJ, Hoogaars WM, et al. Overactive bone morphogenetic protein signaling in heterotopic ossification and Duchenne muscular dystrophy. Cell Mol Life Sci. 2013; 70:407–423.
Article50. Tanaka K, Inoue Y, Hendy GN, et al. Interaction of Tmem119 and the bone morphogenetic protein pathway in the commitment of myoblastic into osteoblastic cells. Bone. 2012; 51:158–167.
Article51. Hisa I, Inoue Y, Hendy GN, et al. Parathyroid hormone-responsive Smad3-related factor, Tmem119, promotes osteoblast differentiation and interacts with the bone morphogenetic protein-Runx2 pathway. J Biol Chem. 2011; 286:9787–9796.
Article52. Tanaka KI, Kaji H, Yamaguchi T, et al. Involvement of the osteoinductive factors, Tmem119 and BMP-2, and the ER stress response PERK-eIF2alpha-ATF4 pathway in the commitment of myoblastic into osteoblastic cells. Calcif Tissue Int. 2013; doi: 10.1007/s00223-013-9828-1.53. Mao L, Yano M, Kawao N, et al. Role of matrix metalloproteinase-10 in the BMP-2 inducing osteoblastic differentiation. Endocr J. 2013; 60:1309–1319.
Article54. Yano M, Kawao N, Tamura Y, et al. A novel factor, Tmem176b, induced by activin-like kinase 2 signal promotes the differentiation of myoblasts into osteoblasts. Exp Clin Endocrinol Diabetes. 2014; 122:7–14.
Article55. Sondag GR, Salihoglu S, Lababidi SL, et al. Osteoactivin induces transdifferentiation of C2C12 myoblasts into osteoblasts. J Cell Physiol. 2013; doi: 10.1002/jcp.24512.
Article56. Wu JY, Aarnisalo P, Bastepe M, et al. Gsalpha enhances commitment of mesenchymal progenitors to the osteoblast lineage but restrains osteoblast differentiation in mice. J Clin Invest. 2011; 121:3492–3504.
Article57. Pignolo RJ, Xu M, Russell E, et al. Heterozygous inactivation of Gnas in adipose-derived mesenchymal progenitor cells enhances osteoblast differentiation and promotes heterotopic ossification. J Bone Miner Res. 2011; 26:2647–2655.
Article58. Zhang RP, Shao JZ, Xiang LX. GADD45A protein plays an essential role in active DNA demethylation during terminal osteogenic differentiation of adipose-derived mesenchymal stem cells. J Biol Chem. 2011; 286:41083–41094.
Article59. Liu Y, Wang L, Kikuiri T, et al. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-gamma and TNF-alpha. Nat Med. 2011; 17:1594–1601.
Article60. Ikeda K, Souma Y, Akakabe Y, et al. Macrophages play a unique role in the plaque calcification by enhancing the osteogenic signals exerted by vascular smooth muscle cells. Biochem Biophys Res Commun. 2012; 425:39–44.
Article61. Kaji H. Menin and bone metabolism. J Bone Miner Metab. 2012; 30:381–387.
Article62. Yano M, Inoue Y, Tobimatsu T, et al. Smad7 inhibits differentiation and mineralization of mouse osteoblastic cells. Endocr J. 2012; 59:653–662.
Article63. Yukita A, Hosoya A, Ito Y, et al. Ubc9 negatively regulates BMP-mediated osteoblastic differentiation in cultured cells. Bone. 2012; 50:1092–1099.
Article64. Ohte S, Kokabu S, Iemura S, et al. Identification and functional analysis of Zranb2 as a novel Smad-binding protein that suppresses BMP signaling. J Cell Biochem. 2012; 113:808–814.
Article65. Wosczyna MN, Biswas AA, Cogswell CA, et al. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res. 2012; 27:1004–1017.
Article66. Tanaka K, Matsumoto E, Higashimaki Y, et al. Role of osteoglycin in the linkage between muscle and bone. J Biol Chem. 2012; 287:11616–11628.
Article67. Tanaka K, Matsumoto E, Higashimaki Y, et al. FAM5C is a soluble osteoblast differentiation factor linking muscle to bone. Biochem Biophys Res Commun. 2012; 418:134–139.
Article68. Cianferotti L, Brandi ML. Muscle-bone interactions: basic and clinical aspects. Endocrine. 2013; doi: 10.1007/s12020-013-0026-8.
Article69. Zhang J, Cheng J, Tu Q, et al. Effects of irisin on bone metabolism and its signal mechanism. In : ASBMR 2013 Annual Meeting; 2013 October 4-7; Baltimore Convention Center. Baltimore, MD: American Society for Bone and Mineral Research.70. Boström P, Wu J, Jedrychowski MP, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012; 481:463–468.
Article71. Abreu EL, Stern M, Brotto M. Bone-muscle interactions: ASBMR Topical Meeting, July 2012. IBMS Bonekey. 2012; 9:239.
Article72. Karsenty G. Osteocalcin and the regulation of muscle mass. In : ASBMR Topical Meeting on Bone and Skeletal Muscle Interactions; 2012 July 16; Westin Crown Center. Kansas City, MO: American Society for Bone and Mineral Research.73. Juffer P, Jaspers RT, Lips P, et al. Expression of muscle anabolic and metabolic factors in mechanically loaded MLO-Y4 osteocytes. Am J Physiol Endocrinol Metab. 2012; 302:E389–E395.
Article74. Jähn K, Lara-Castillo N, Brotto L, et al. Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of beta-catenin. Eur Cell Mater. 2012; 24:197–209.75. Rodgers BD, Garikipati DK. Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocr Rev. 2008; 29:513–534.
Article76. Arounleut P, Bialek P, Elsalanty M, et al. A myostatin inhibitor (propeptide-Fc) increases muscle mass but does not alter bone density or strength in aged mice. In : ASBMR 2013 Annual Meeting; 2013 October 4-7; Baltimore Convention Center. Baltimore, MD: American Society for Bone and Mineral Research.77. Sassoli C, Pini A, Chellini F, et al. Bone marrow mesenchymal stromal cells stimulate skeletal myoblast proliferation through the paracrine release of VEGF. PLoS One. 2012; 7:e37512.
Article78. Mo C, Romero-Suarez S, Bonewald L, et al. Prostaglandin E2: from clinical applications to its potential role in bone-muscle crosstalk and myogenic differentiation. Recent Pat Biotechnol. 2012; 6:223–229.
Article79. Gorski J, Huffman NT, Brotto L, et al. Potential role of leptin and BMP2 in osteocyte regulation of muscle mass and function in the adult skeleton and with age. In : ASBMR 2013 Annual Meeting; 2013 October 4-7; Baltimore Convention Center. Baltimore, MD: American Society for Bone and Mineral Research.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diverse Abnormal Body Composition Phenotypes: Interaction Between Muscle, Fat, and Bone
- Importance of Sclerostin as Bone-Muscle Mediator Crosstalk
- Anatomy and variations of digastric muscle
- Food Security Moderates the Relationships of Muscle Mass with Metabolic Syndrome and Insulin Resistance
- Maternal-Fetal Interaction Belief and Maternal-Fetal Interaction