Allergy Asthma Immunol Res.
2016 May;8(3):239-245. 10.4168/aair.2016.8.3.239.
Antibody Production, Anaphylactic Signs, and T-Cell Responses Induced by Oral Sensitization With Ovalbumin in BALB/c and C3H/HeOuJ Mice
- Affiliations
-
- 1Instituto de Investigacion en Ciencias de la Alimentacion (CIAL, CSIC-UAM), Nicolas Cabrera, Madrid, Spain. e.molina@csic.es
- KMID: 2391044
- DOI: http://doi.org/10.4168/aair.2016.8.3.239
Abstract
- PURPOSE
Two mouse strains, BALB/c and C3H/HeOuJ, broadly used in the field of food allergy, were compared for the evaluation of the allergenic potential of ovalbumin (OVA).
METHODS
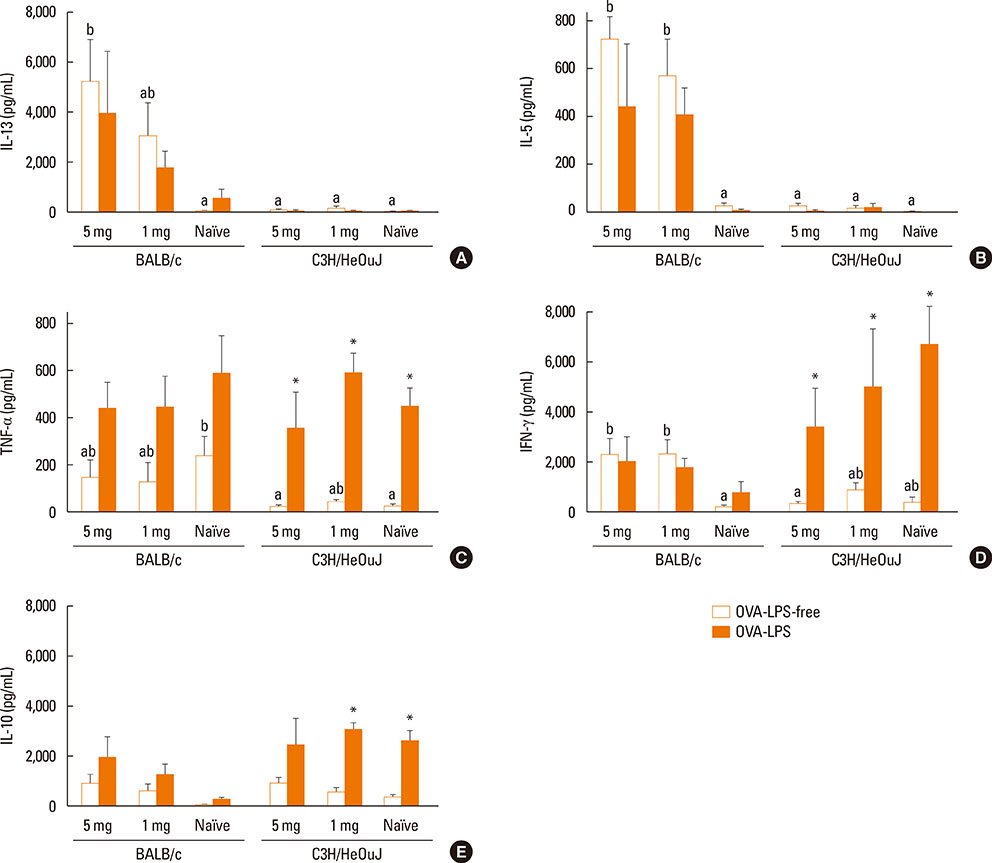
Sensitization was made by administering 2 different OVA doses (1 and 5 mg), with cholera toxin as Th2-polarizing adjuvant. Antibody levels, severity of anaphylaxis, and Th1 and Th2 responses induced by the allergen were assessed. In addition, because the mice selected had functional toll-like receptor 4, the influence of contamination with lipopolysaccharide (LPS) on the immunostimulating capacity of OVA on spleen cells was also evaluated.
RESULTS
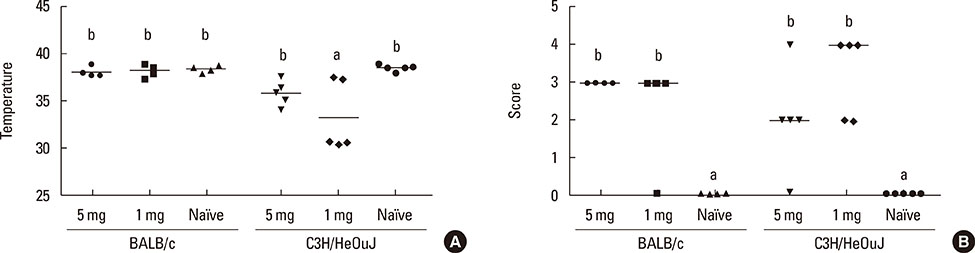
Both strains exhibited similar susceptibility to OVA sensitization. The 2 protein doses generated similar OVA-specific IgE and IgG1 levels in both strains, whereas C3H/HeOuJ mice produced significantly more IgG2a. Oral challenge provoked more severe manifestations in C3H/HeOuJ mice as indicated by the drop in body temperature and the severity of the anaphylactic scores. Stimulation of splenocytes with OVA led to significantly higher levels of Th2 and Th1 cytokines in BALB/c, and these were less affected by protein contamination with LPS.
CONCLUSIONS
The antibody and cytokine levels induced by OVA in BALB/c mice and the observation that BALB/c spleen cell cultures were more resistant than those of C3H/HeOuJ mice to the stimulus of LPS make this strain prone to exhibit Th2-mediated food allergic reactions and very adequate for the study of the features of OVA that make it allergenic.
MeSH Terms
-
Anaphylaxis
Animals
Antibody Formation*
Body Temperature
Cell Culture Techniques
Cholera Toxin
Cytokines
Food Hypersensitivity
Hypersensitivity
Immunoglobulin E
Immunoglobulin G
Mice*
Ovalbumin*
Ovum
Spleen
T-Lymphocytes*
Toll-Like Receptor 4
Cholera Toxin
Cytokines
Immunoglobulin E
Immunoglobulin G
Ovalbumin
Toll-Like Receptor 4
Figure
Reference
-
1. Oyoshi MK, Oettgen HC, Chatila TA, Geha RS, Bryce PJ. Food allergy: insights into etiology, prevention, and treatment provided by murine models. J Allergy Clin Immunol. 2014; 133:309–317.2. Berin MC, Shreffler WG. T(H)2 adjuvants: implications for food allergy. J Allergy Clin Immunol. 2008; 121:1311–1320.3. Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007; 120:506–515.4. Berin MC, Zheng Y, Domaradzki M, Li XM, Sampson HA. Role of TLR4 in allergic sensitization to food proteins in mice. Allergy. 2006; 61:64–71.5. van Wijk F, Nierkens S, Hassing I, Feijen M, Koppelman SJ, de Jong GA, et al. The effect of the food matrix on in vivo immune responses to purified peanut allergens. Toxicol Sci. 2005; 86:333–341.6. Roth-Walter F, Berin MC, Arnaboldi P, Escalante CR, Dahan S, Rauch J, et al. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer's patches. Allergy. 2008; 63:882–890.7. Martos G, Lopez-Exposito I, Bencharitiwong R, Berin MC, Nowak-Węgrzyn A. Mechanisms underlying differential food allergy response to heated egg. J Allergy Clin Immunol. 2011; 127:990–997.e1-2.8. Dearman RJ, Kimber I. Animal models of protein allergenicity: potential benefits, pitfalls and challenges. Clin Exp Allergy. 2009; 39:458–468.9. Morafo V, Srivastava K, Huang CK, Kleiner G, Lee SY, Sampson HA, et al. Genetic susceptibility to food allergy is linked to differential TH2-TH1 responses in C3H/HeJ and BALB/c mice. J Allergy Clin Immunol. 2003; 111:1122–1128.10. Mine Y, Yang M. Recent advances in the understanding of egg allergens: basic, industrial, and clinical perspectives. J Agric Food Chem. 2008; 56:4874–4900.11. Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007; 120:638–646.12. Hilmenyuk T, Bellinghausen I, Heydenreich B, Ilchmann A, Toda M, Grabbe S, et al. Effects of glycation of the model food allergen ovalbumin on antigen uptake and presentation by human dendritic cells. Immunology. 2010; 129:437–445.13. Untersmayr E, Diesner SC, Oostingh GJ, Selzle K, Pfaller T, Schultz C, et al. Nitration of the egg-allergen ovalbumin enhances protein allergenicity but reduces the risk for oral sensitization in a murine model of food allergy. PLoS One. 2010; 5:e14210.14. Zuercher AW, Holvoet S, Weiss M, Mercenier A. Polyphenol-enriched apple extract attenuates food allergy in mice. Clin Exp Allergy. 2010; 40:942–950.15. Dearman RJ, Beresford L, Foster ES, McClain S, Kimber I. Characterization of the allergenic potential of proteins: an assessment of the kiwifruit allergen actinidin. J Appl Toxicol. 2014; 34:489–497.16. Brix S, Bovetto L, Fritsché R, Barkholt V, Frøkiaer H. Immunostimulatory potential of β-lactoglobulin preparations: effects caused by endotoxin contamination. J Allergy Clin Immunol. 2003; 112:1216–1222.17. López-Expósito I, Chicón R, Belloque J, López-Fandiño R, Berin MC. In vivo methods for testing allergenicity show that high hydrostatic pressure hydrolysates of β-lactoglobulin are immunologically inert. J Dairy Sci. 2012; 95:541–548.18. Li XM, Schofield BH, Huang CK, Kleiner GI, Sampson HA. A murine model of IgE-mediated cow's milk hypersensitivity. J Allergy Clin Immunol. 1999; 103:206–214.19. Perrier C, Thierry AC, Mercenier A, Corthésy B. Allergen-specific antibody and cytokine responses, mast cell reactivity and intestinal permeability upon oral challenge of sensitized and tolerized mice. Clin Exp Allergy. 2010; 40:153–162.20. Lee SY, Huang CK, Zhang TF, Schofield BH, Burks AW, Bannon GA, et al. Oral administration of IL-12 suppresses anaphylactic reactions in a murine model of peanut hypersensitivity. Clin Immunol. 2001; 101:220–228.21. Thomas K, Herouet C, Bannon G, Ladics G, MacIntosh S, Privalle L, et al. Evaluation of IP mouse models for assessing the allergenic potential of proteins. J Allergy Clin Immunol. 2005; 115:S250.22. Ladics GS, Knippels LM, Penninks AH, Bannon GA, Goodman RE, Herouet-Guicheney C. Review of animal models designed to predict the potential allergenicity of novel proteins in genetically modified crops. Regul Toxicol Pharmacol. 2010; 56:212–224.23. Berin MC, Sampson HA. Mucosal immunology of food allergy. Curr Biol. 2013; 23:R389–R400.24. Smit JJ, Willemsen K, Hassing I, Fiechter D, Storm G, van Bloois L, et al. Contribution of classic and alternative effector pathways in peanut-induced anaphylactic responses. PLoS One. 2011; 6:e28917.25. Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH, et al. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000; 106:150–158.26. Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, et al. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988; 334:255–258.27. Yang M, Yang C, Mine Y. Multiple T cell epitope peptides suppress allergic responses in an egg allergy mouse model by the elicitation of forkhead box transcription factor 3- and transforming growth factor-beta-associated mechanisms. Clin Exp Allergy. 2010; 40:668–678.28. Diesner SC, Knittelfelder R, Krishnamurthy D, Pali-Schöll I, Gajdzik L, Jensen-Jarolim E, et al. Dose-dependent food allergy induction against ovalbumin under acid-suppression: a murine food allergy model. Immunol Lett. 2008; 121:45–51.29. Shindo T, Kanazawa Y, Saito Y, Kojima K, Ohsawa M, Teshima R. Effective induction of oral anaphylaxis to ovalbumin in mice sensitized by feeding of the antigen with aid of oil emulsion and salicylate. J Toxicol Sci. 2012; 37:307–315.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intestinal mastocytosis and goblet cell hyperplasia in BALB/c and C3H mice infected with Neodiplostomum seoulense
- Eosinophils are Required for Immune Responses Induced by Oral Immunization
- Effects of Bacillus Calmette-Guerin (BCG) Vaccination according to Time Point of Administration on Asthma in a Murine Model
- Effect of DHEA Ingestion on Atopic Dermatitis-like Lesion in BALB/c Mice Sensitized by Ovalbumin
- Induction of Active Systemic Anaphylaxis and Immunological Aspects in Mice Sensitized with House Dust Mite