Blood Res.
2017 Sep;52(3):207-211. 10.5045/br.2017.52.3.207.
Efficacy of eculizumab in paroxysmal nocturnal hemoglobinuria patients with or without aplastic anemia: prospective study of a Korean PNH cohort
- Affiliations
-
- 1Department of Internal Medicine, Korea University Guro Hospital, Seoul, Korea.
- 2Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Chungnam National University, Daejeon, Korea.
- 5Department of Internal Medicine, Asan Medical Center, University of Ulsan, College of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Dong-A University Hospital, Busan, Korea.
- 7Department of Internal Medicine, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 8Department of Internal Medicine, SoonChunHyang University Hospital, Seoul, Korea.
- 9Department of Internal Medicine, Pusan National University Hospital, Busan, Korea.
- 10Department of Internal Medicine, Ulsan University Hospital, Ulsan, Korea.
- 11Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea.
- 12Department of Internal Medicine, Youngnam University Hospital, Daegu, Korea.
- 13Department of Internal Medicine, National Cancer Center, Goyang, Korea.
- 14Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 15Department of Internal Medicine, St. Vincent Hospital, The Catholic University of Korea, Suwon, Korea.
- 16Department of Internal Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea. jwlee@catholic.ac.kr
- KMID: 2390983
- DOI: http://doi.org/10.5045/br.2017.52.3.207
Abstract
- BACKGROUND
Patients with paroxysmal nocturnal hemoglobinuria (PNH) often have concurrent aplastic anemia (AA). This study aimed to determine whether eculizumab-treated patients show clinical benefit regardless of concurrent AA.
METHODS
We analyzed 46 PNH patients ≥18 years of age who were diagnosed by flow cytometry and treated with eculizumab for more than 6 months in the prospective Korean PNH registry. Patients were categorized into two groups: PNH patients with concurrent AA (PNH/AA, N=27) and without AA (classic PNH, N=19). Biochemical indicators of intravascular hemolysis, hematological laboratory values, transfusion requirement, and PNH-associated complications were assessed at baseline and every 6 months after initiation of eculizumab treatment.
RESULTS
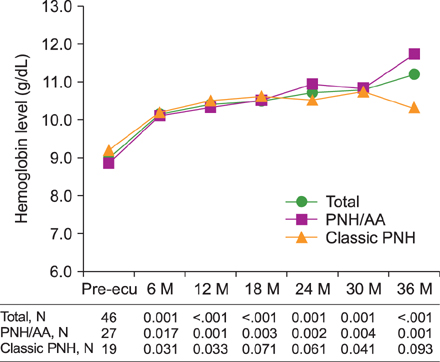
The median patient age was 46 years and median duration of eculizumab treatment was 34 months. Treatment with eculizumab induced rapid inhibition of hemolysis. At 6-month follow-up, LDH decreased to near normal levels in all patients; this effect was maintained until the 36-month follow-up regardless of concurrent AA. Transfusion independence was achieved by 53.3% of patients within the first 6 months of treatment and by 90.9% after 36 months of treatment. The mean number of RBC units transfused was significantly reduced, from 8.5 units during the 6 months prior to initiation of eculizumab to 1.6 units in the first 6 months of treatment, for the total study population; this effect was similar in both PNH/AA and classic PNH.
CONCLUSION
This study demonstrated that eculizumab is beneficial in the management of patients with PNH/AA, similar to classic PNH.
MeSH Terms
Figure
Cited by 1 articles
-
Detection of GPI-deficient Cells in Paroxysmal Nocturnal Hemoglobinuria Patients Using Flow Cytometry: Current Status in Korea and Proposal for Testing Guidelines
Hyun-Young Kim, Min-Sun Kim, Saeam Shin, Ari Ahn, Chan-Jeong Park, Jaewoo Song, Duck Cho, Sang Mee Hwang, Miyoung Kim
Lab Med Online. 2024;14(2):75-81. doi: 10.47429/lmo.2024.14.2.75.
Reference
-
1. Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995; 333:1253–1258.
Article2. Schrezenmeier H, Muus P, Socié G, et al. Baseline characteristics and disease burden in patients in the International Paroxysmal Nocturnal Hemoglobinuria Registry. Haematologica. 2014; 99:922–929.
Article3. Hillmen P, Muus P, Röth A, et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2013; 162:62–73.
Article4. Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005; 106:3699–3709.
Article5. Kelly RJ, Hill A, Arnold LM, et al. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011; 117:6786–6792.
Article6. Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006; 355:1233–1243.
Article7. Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008; 111:1840–1847.
Article8. Kanakura Y, Ohyashiki K, Shichishima T, et al. Safety and efficacy of the terminal complement inhibitor eculizumab in Japanese patients with paroxysmal nocturnal hemoglobinuria: the AEGIS clinical trial. Int J Hematol. 2011; 93:36–46.
Article9. Lee JW, Jang JH, Kim JS, et al. Clinical signs and symptoms associated with increased risk for thrombosis in patients with paroxysmal nocturnal hemoglobinuria from a Korean Registry. Int J Hematol. 2013; 97:749–757.
Article10. Al-Ani F, Chin-Yee I, Lazo-Langner A. Eculizumab in the management of paroxysmal nocturnal hemoglobinuria: patient selection and special considerations. Ther Clin Risk Manag. 2016; 12:1161–1170.11. Hillmen P, Elebute M, Kelly R, et al. Long-term effect of the complement inhibitor eculizumab on kidney function in patients with paroxysmal nocturnal hemoglobinuria. Am J Hematol. 2010; 85:553–559.
Article12. Jang JH, Kim JS, Yoon SS, et al. Predictive factors of mortality in population of patients with paroxysmal nocturnal hemoglobinuria (PNH): Results from a Korean PNH Registry. J Korean Med Sci. 2016; 31:214–221.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CD55 and CD59 : New Diagnostic Tests for Paroxysmal Nocturnal Hemoglobinuria
- Anesthetic and Perioperative Management of Patient with Paroxysmal Nocturnal Hemoglobinuria
- Paroxysmal Nocturnal Hemoglobinuria Presenting as Recurrent Jejunitis
- A Successful Planned Pregnancy and Delivery with Eculizumab Maintenance in a Woman with Paroxysmal Nocturnal Hemoglobinuria
- The use of the complement inhibitor eculizumab (Soliris(R)) for treating Korean patients with paroxysmal nocturnal hemoglobinuria