Blockade of Kv1.5 channels by the antidepressant drug sertraline
- Affiliations
-
- 1Department of Pharmacology, Institute for Medical Sciences, Chonbuk National University Medical School, Jeonju 54097, Korea. bhchoi@jbnu.ac.kr
- 2Department of Physiology, Medical Research Center, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- KMID: 2388676
- DOI: http://doi.org/10.4196/kjpp.2016.20.2.193
Abstract
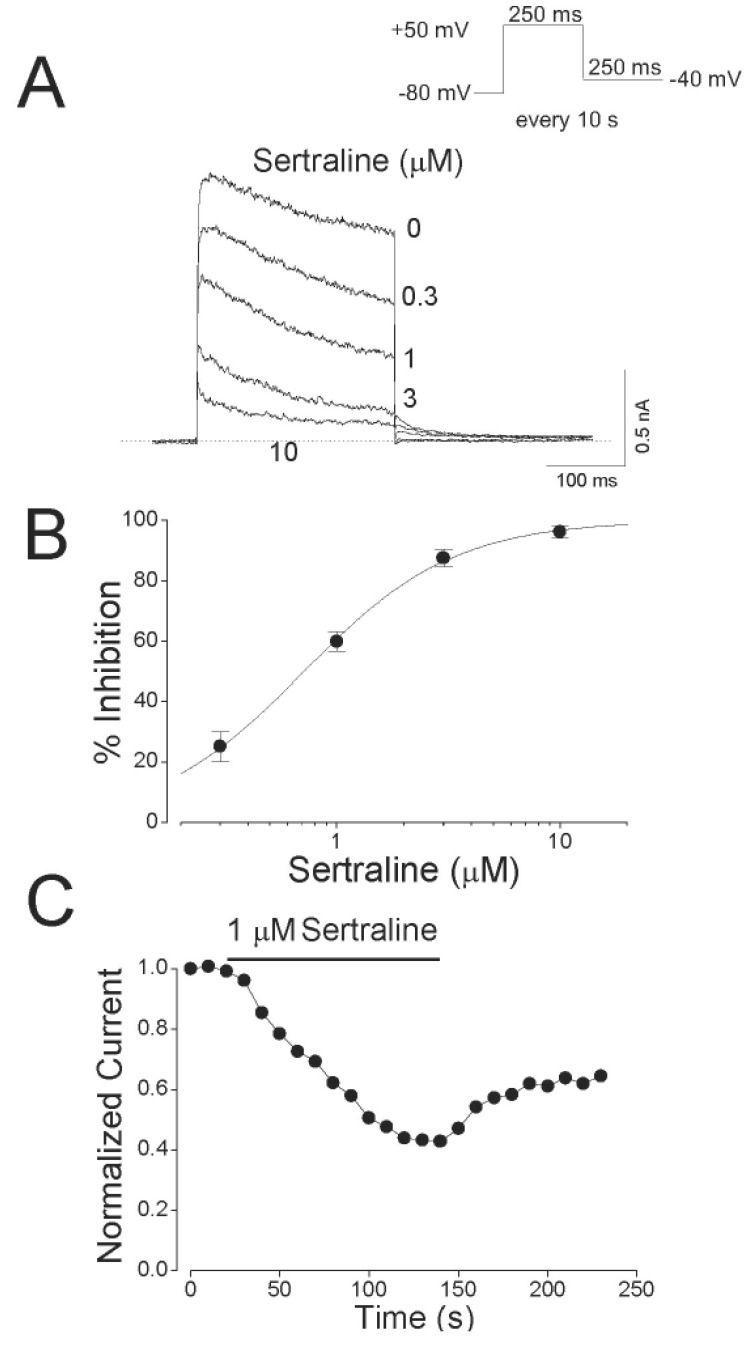
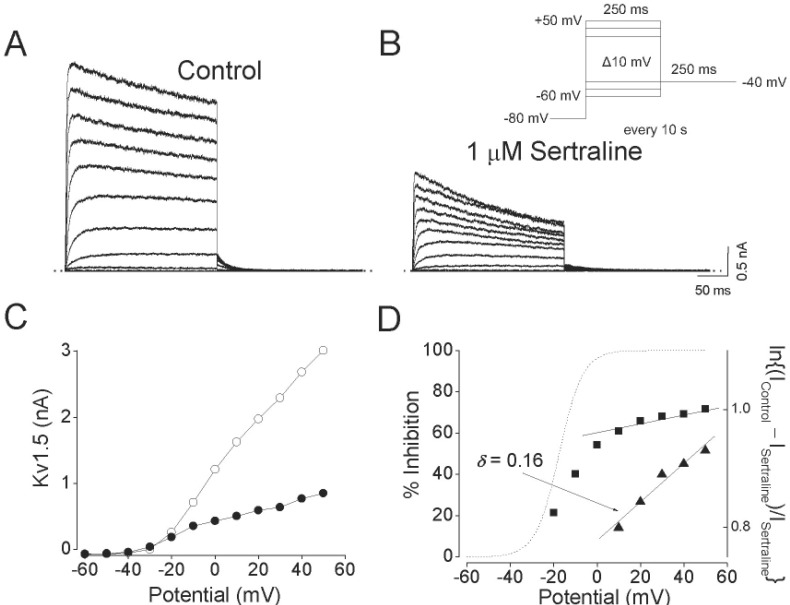
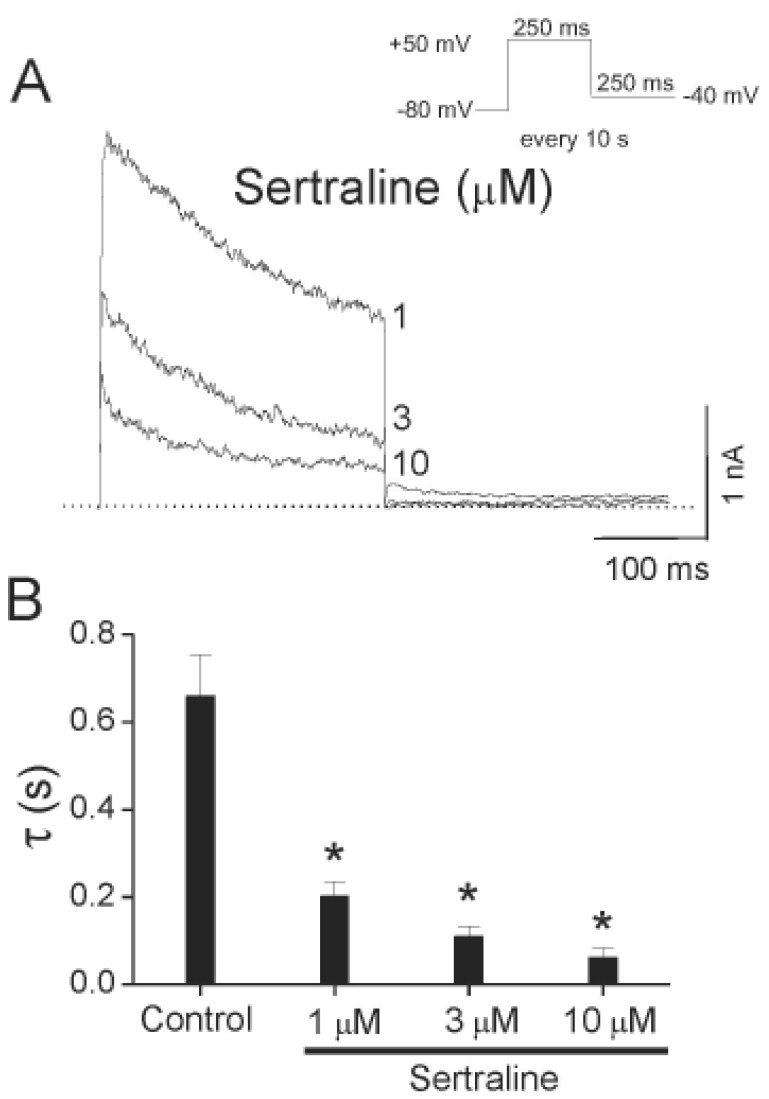
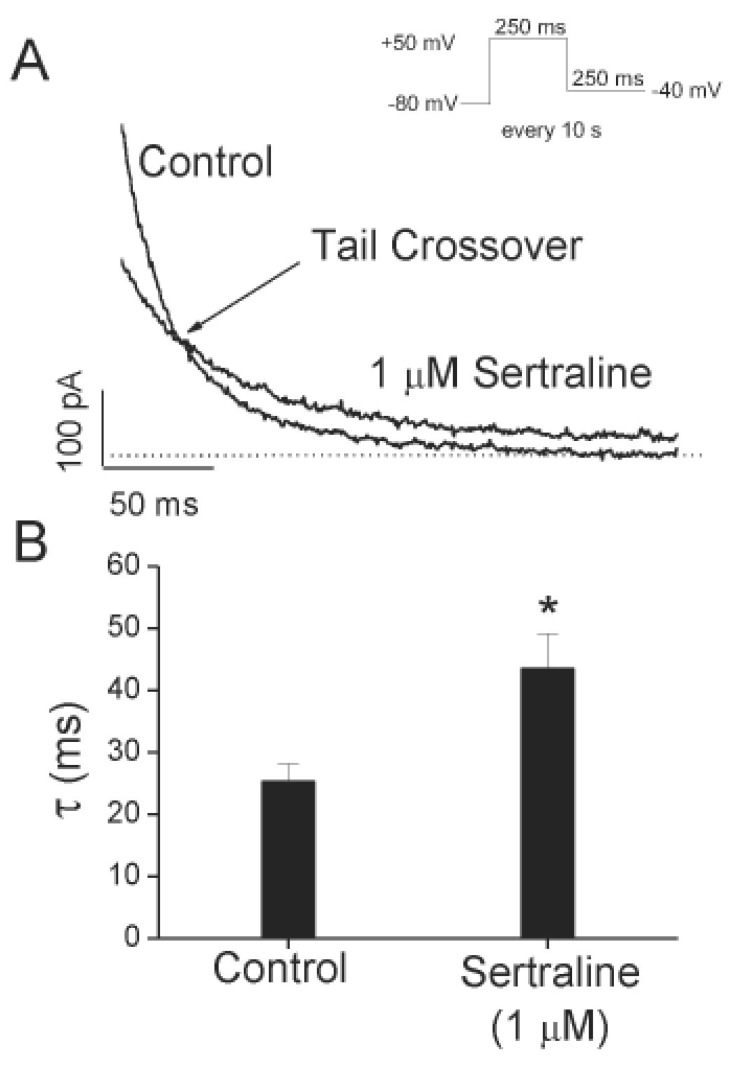
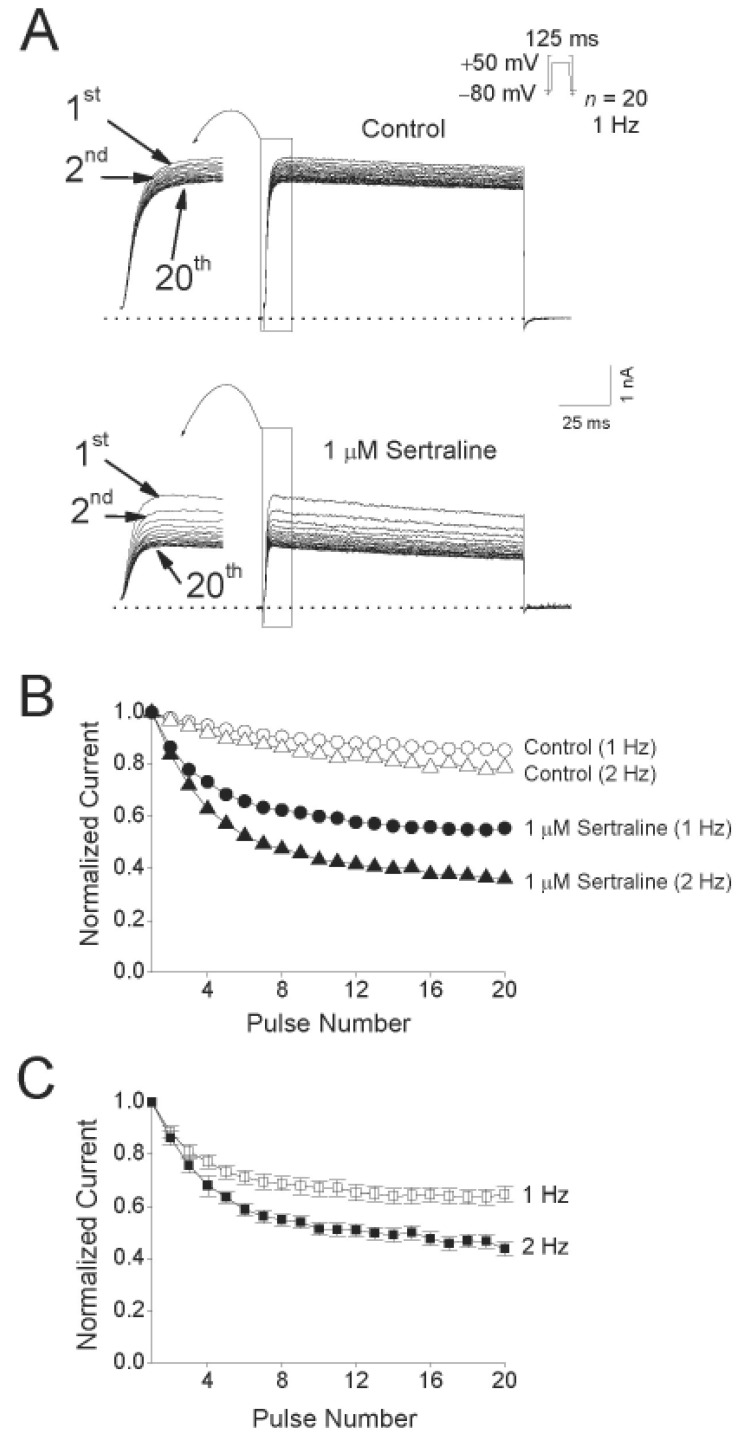
- Sertraline, a selective serotonin reuptake inhibitor (SSRI), has been reported to lead to cardiac toxicity even at therapeutic doses including sudden cardiac death and ventricular arrhythmia. And in a SSRI-independent manner, sertraline has been known to inhibit various voltage-dependent channels, which play an important role in regulation of cardiovascular system. In the present study, we investigated the action of sertraline on Kv1.5, which is one of cardiac ion channels. The eff ect of sertraline on the cloned neuronal rat Kv1.5 channels stably expressed in Chinese hamster ovary cells was investigated using the whole-cell patch-clamp technique. Sertraline reduced Kv1.5 whole-cell currents in a reversible concentration-dependent manner, with an IC50 value and a Hill coefficient of 0.71 microM and 1.29, respectively. Sertraline accelerated the decay rate of inactivation of Kv1.5 currents without modifying the kinetics of current activation. The inhibition increased steeply between -20 and 0 mV, which corresponded with the voltage range for channel opening. In the voltage range positive to +10 mV, inhibition displayed a weak voltage dependence, consistent with an electrical distance delta of 0.16. Sertraline slowed the deactivation time course, resulting in a tail crossover phenomenon when the tail currents, recorded in the presence and absence of sertraline, were superimposed. Inhibition of Kv1.5 by sertraline was use-dependent. The present results suggest that sertraline acts on Kv1.5 currents as an open-channel blocker.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Open channel block of Kv1.4 potassium channels by aripiprazole
Jeaneun Park, Kwang-Hyun Cho, Hong Joon Lee, Jin-Sung Choi, Duck-Joo Rhie
Korean J Physiol Pharmacol. 2020;24(6):545-553. doi: 10.4196/kjpp.2020.24.6.545.Facilitation of serotonin-induced contraction of rat mesenteric artery by ketamine
Sang Woong Park, Hyun Ju Noh, Jung Min Kim, Bokyung Kim, Sung-Il Cho, Yoon Soo Kim, Nam Sik Woo, Sung Hun Kim, Young Min Bae
Korean J Physiol Pharmacol. 2016;20(6):605-611. doi: 10.4196/kjpp.2016.20.6.605.Nortriptyline, a tricyclic antidepressant, inhibits voltage-dependent K+ channels in coronary arterial smooth muscle cells
Sung Eun Shin, Hongliang Li, Han Sol Kim, Hye Won Kim, Mi Seon Seo, Kwon-Soo Ha, Eun-Taek Han, Seok-Ho Hong, Amy L. Firth, Il-Whan Choi, Young Min Bae, Won Sun Park
Korean J Physiol Pharmacol. 2017;21(2):225-232. doi: 10.4196/kjpp.2017.21.2.225.The antidiabetic drug rosiglitazone blocks Kv1.5 potassium channels in an open state
Hyang Mi Lee, Sang June Hahn, Bok Hee Choi
Korean J Physiol Pharmacol. 2022;26(2):135-144. doi: 10.4196/kjpp.2022.26.2.135.
Reference
-
1. Grimsley SR, Jann MW. Paroxetine, sertraline, and fluvoxamine: new selective serotonin reuptake inhibitors. Clin Pharm. 1992; 11:930–957. PMID: 1464219.2. Leonard CE, Bilker WB, Newcomb C, Kimmel SE, Hennessy S. Antidepressants and the risk of sudden cardiac death and ventricular arrhythmia. Pharmacoepidemiol Drug Saf. 2011; 20:903–913. PMID: 21796718.
Article3. Lusetti M, Licata M, Silingardi E, Reggiani Bonetti L, Palmiere C. Cardiac toxicity in selective serotonin reuptake inhibitor users. Am J Forensic Med Pathol. 2015; 36:293–297. PMID: 26448056.
Article4. Aldana BI, Sitges M. Sertraline inhibits pre-synaptic Na+ channel-mediated responses in hippocampus-isolated nerve endings. J Neurochem. 2012; 121:197–205. PMID: 22288826.5. Becker B, Morel N, Vanbellinghen AM, Lebrun P. Blockade of calcium entry in smooth muscle cells by the antidepressant imipramine. Biochem Pharmacol. 2004; 68:833–842. PMID: 15294446.
Article6. Lee HA, Kim KS, Hyun SA, Park SG, Kim SJ. Wide spectrum of inhibitory effects of sertraline on cardiac ion channels. Korean J Physiol Pharmacol. 2012; 16:327–332. PMID: 23118556.
Article7. Ohno Y, Hibino H, Lossin C, Inanobe A, Kurachi Y. Inhibition of astroglial Kir4.1 channels by selective serotonin reuptake inhibitors. Brain Res. 2007; 1178:44–51. PMID: 17920044.
Article8. Wang GK, Mitchell J, Wang SY. Block of persistent late Na+ currents by antidepressant sertraline and paroxetine. J Membr Biol. 2008; 222:79–90. PMID: 18418539.9. Fonseca-Magalhães PA, Sousa DF, de Siqueira RJ, Jorge RJ, Meneses GC, Alves RS, Monteiro HS, Magalhães PJ, Martins AM. Inhibitory effects of sertraline in rat isolated perfused kidneys and in isolated ring preparations of rat arteries. J Pharm Pharmacol. 2011; 63:1186–1194. PMID: 21827491.
Article10. Colatsky TJ, Follmer CH, Starmer CF. Channel specificity in antiarrhythmic drug action. Mechanism of potassium channel block and its role in suppressing and aggravating cardiac arrhythmias. Circulation. 1990; 82:2235–2242. PMID: 2242545.
Article11. Schumacher SM, McEwen DP, Zhang L, Arendt KL, Van Genderen KM, Martens JR. Antiarrhythmic drug-induced internalization of the atrial-specific k+ channel kv1.5. Circ Res. 2009; 104:1390–1398. PMID: 19443837.12. Wang Z, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 1993; 73:1061–1076. PMID: 8222078.13. Swanson R, Marshall J, Smith JS, Williams JB, Boyle MB, Folander K, Luneau CJ, Antanavage J, Oliva C, Buhrow SA, Bennet C, Stein RB, Kaczmarek LK. Cloning and expression of cDNA and genomic clones encoding three delayed rectifier potassium channels in rat brain. Neuron. 1990; 4:929–939. PMID: 2361015.
Article14. Li GR, Feng J, Yue L, Carrier M, Nattel S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ Res. 1996; 78:689–696. PMID: 8635226.15. Choi BH, Choi JS, Jeong SW, Hahn SJ, Yoon SH, Jo YH, Kim MS. Direct block by bisindolylmaleimide of rat Kv1.5 expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 2000; 293:634–640. PMID: 10773038.16. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981; 391:85–100. PMID: 6270629.
Article17. Snyders DJ, Yeola SW. Determinants of antiarrhythmic drug action. Electrostatic and hydrophobic components of block of the human cardiac hKv1.5 channel. Circ Res. 1995; 77:575–583. PMID: 7641327.18. Woodhull AM. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973; 61:687–708. PMID: 4541078.
Article19. Delpón E, Valenzuela C, Gay P, Franqueza L, Snyders DJ, Tamargo J. Block of human cardiac Kv1.5 channels by loratadine: voltage-, time- and use-dependent block at concentrations above therapeutic levels. Cardiovasc Res. 1997; 35:341–350. PMID: 9349397.
Article20. Sung MJ, Hahn SJ, Choi BH. Effect of psoralen on the cloned Kv3.1 currents. Arch Pharm Res. 2009; 32:407–412. PMID: 19387585.
Article21. Valenzuela C, Delpón E, Franqueza L, Gay P, Pérez O, Tamargo J, Snyders DJ. Class III antiarrhythmic effects of zatebradine. Time-, state-, use-, and voltage-dependent block of hKv1.5 channels. Circulation. 1996; 94:562–570. PMID: 8759103.22. Franqueza L, Valenzuela C, Delpón E, Longobardo M, Caballero R, Tamargo J. Effects of propafenone and 5-hydroxy-propafenone on hKv1.5 channels. Br J Pharmacol. 1998; 125:969–978. PMID: 9846634.
Article23. Snyders J, Knoth KM, Roberds SL, Tamkun MM. Time-, voltage-, and state-dependent block by quinidine of a cloned human cardiac potassium channel. Mol Pharmacol. 1992; 41:322–330. PMID: 1538710.24. Choi JS, Hahn SJ, Rhie DJ, Yoon SH, Jo YH, Kim MS. Mechanism of fluoxetine block of cloned voltage-activated potassium channel Kv1.3. J Pharmacol Exp Ther. 1999; 291:1–6. PMID: 10490879.25. Lee HM, Hahn SJ, Choi BH. Open channel block of Kv1.5 currents by citalopram. Acta Pharmacol Sin. 2010; 31:429–435. PMID: 20228830.
Article26. Wu J, Ding WG, Matsuura H, Tsuji K, Zang WJ, Horie M. Inhibitory actions of the phosphatidylinositol 3-kinase inhibitor LY294002 on the human Kv1.5 channel. Br J Pharmacol. 2009; 156:377–387. PMID: 19154427.
Article27. Valenti TW Jr, Perez-Hurtado P, Chambliss CK, Brooks BW. Aquatic toxicity of sertraline to Pimephales promelas at environmentally relevant surface water pH. Environ Toxicol Chem. 2009; 28:2685–2694. PMID: 19663538.
Article28. Mauri MC, Laini V, Cerveri G, Scalvini ME, Volonteri LS, Regispani F, Malvini L, Manfré S, Boscati L, Panza G. Clinical outcome and tolerability of sertraline in major depression: a study with plasma levels. Prog Neuropsychopharmacol Biol Psychiatry. 2002; 26:597–601. PMID: 11999914.29. Aréchiga IA, Barrio-Echavarria GF, Rodríguez-Menchaca AA, Moreno-Galindo EG, Decher N, Tristani-Firouzi M, Sánchez-Chapula JA, Navarro-Polanco RA. Kv1.5 open channel block by the antiarrhythmic drug disopyramide: molecular determinants of block. J Pharmacol Sci. 2008; 108:49–55. PMID: 18818480.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The antidiabetic drug rosiglitazone blocks Kv1.5 potassium channels in an open state
- Wide Spectrum of Inhibitory Effects of Sertraline on Cardiac Ion Channels
- Blockade of Kv1.5 by paroxetine, an antidepressant drug
- Antidepressant and the Quality of Life of Depressive Patient
- Effects of 3,3′,4,4′,5-pentachlorobiphenyl on human Kv1.3 and Kv1.5 channels