Korean J Physiol Pharmacol.
2022 Mar;26(2):135-144. 10.4196/kjpp.2022.26.2.135.
The antidiabetic drug rosiglitazone blocks Kv1.5 potassium channels in an open state
- Affiliations
-
- 1Department of Pharmacology, Institute for Medical Sciences, Jeonbuk National University Medical School, Jeonju 54097, Korea
- 2Department of Physiology, Medical Research Center, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea
- KMID: 2526732
- DOI: http://doi.org/10.4196/kjpp.2022.26.2.135
Abstract
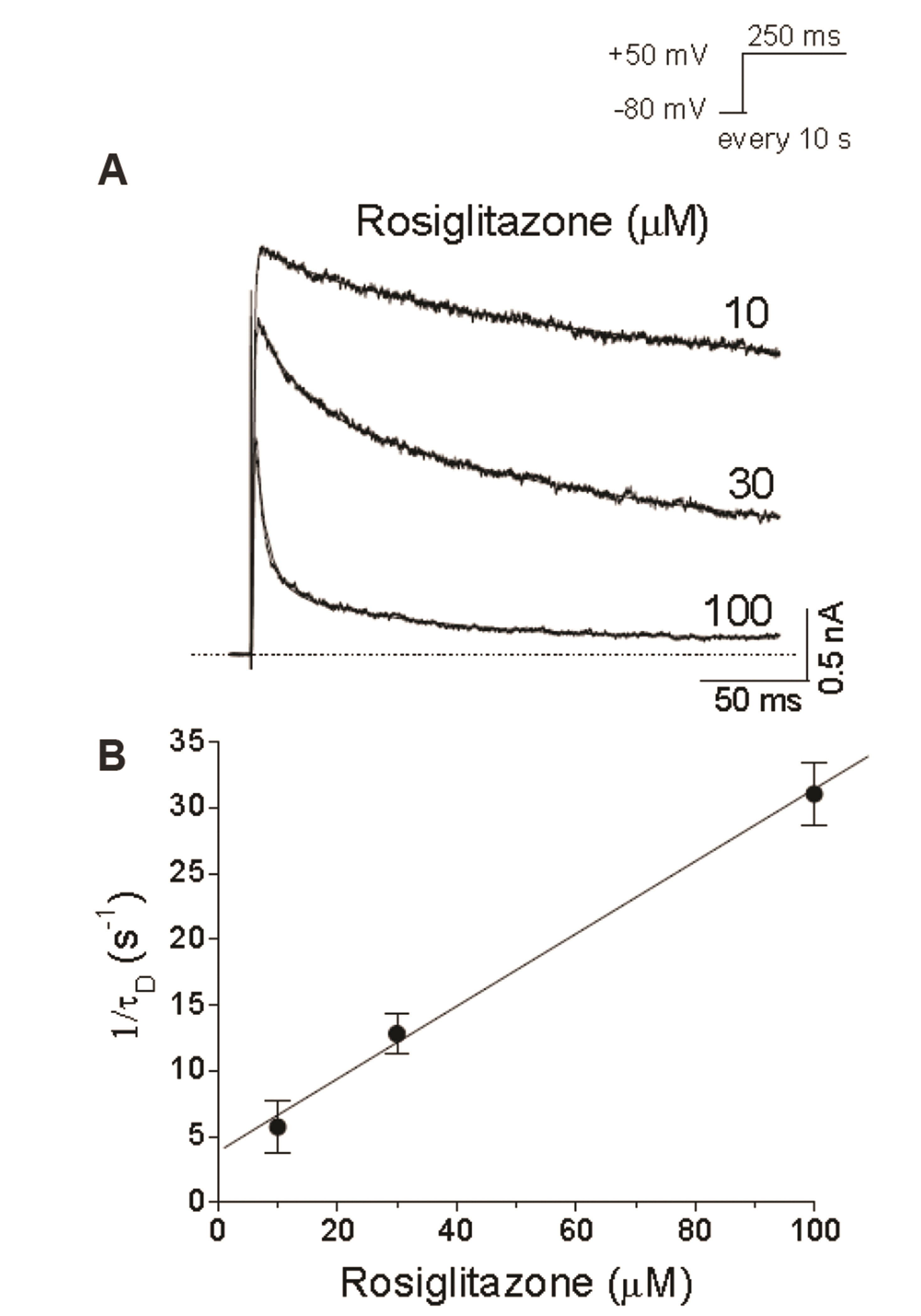
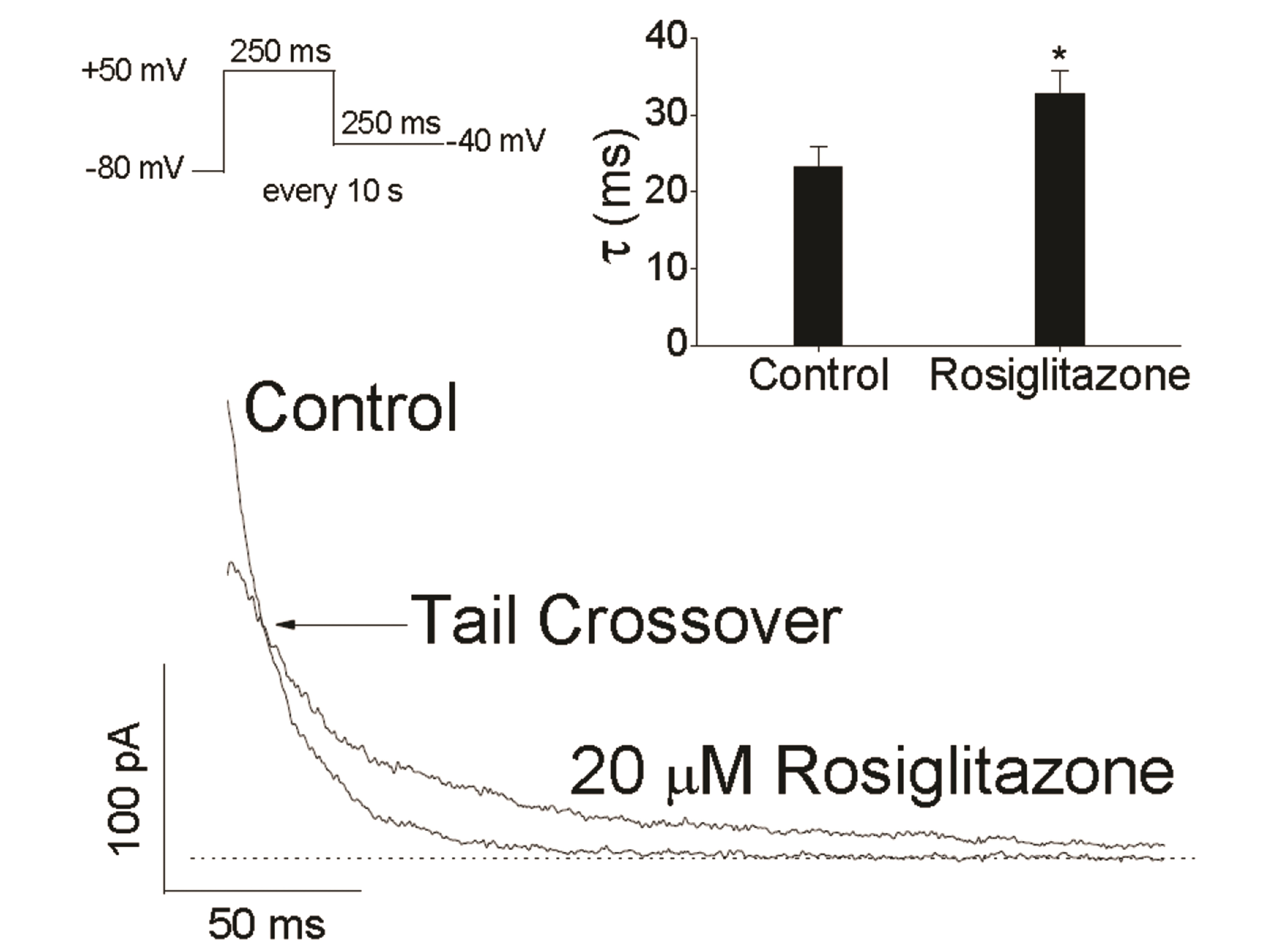
- An antidiabetic drug, rosiglitazone is a member of the drug class of thiazolidinedione. Although restrictions on use due to the possibility of heart toxicity have been removed, it is still a drug that is concerned about side effects on the heart. We here examined, using Chinese hamster ovary cells, the action of rosiglitazone on Kv1.5 channels, which is a major determinant of the duration of cardiac action potential. Rosiglitazone rapidly and reversibly inhibited Kv1.5 currents in a concentrationdependent manner (IC 50 = 18.9 µM) and accelerated the decay of Kv1.5 currents without modifying the activation kinetics. In addition, the deactivation of Kv1.5 current, assayed with tail current, was slowed by the drug. All of the results as well as the usedependence of the rosiglitazone-mediated blockade indicate that rosiglitazone acts on Kv1.5 channels as an open channel blocker. This study suggests that the cardiac side effects of rosiglitazone might be mediated in part by suppression of Kv1.5 channels, and therefore, raises a concern of using the drug for diabetic therapeutics.
Figure
Reference
-
1. Deeks ED, Keam SJ. 2007; Rosiglitazone: a review of its use in type 2 diabetes mellitus. Drugs. 67:2747–2779. DOI: 10.2165/00003495-200767180-00008. PMID: 18062722.2. Wagstaff AJ, Goa KL. 2002; Rosiglitazone: a review of its use in the management of type 2 diabetes mellitus. Drugs. 62:1805–1837. DOI: 10.2165/00003495-200262120-00007. PMID: 12149047.3. Nissen SE, Wolski K. 2007; Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 356:2457–2471. Erratum in: N Engl J Med. 2007;357:100. DOI: 10.1056/NEJMoa072761. PMID: 17517853.
Article4. Lebovitz HE. 2019; Thiazolidinediones: the forgotten diabetes medications. Curr Diab Rep. 19:151. DOI: 10.1007/s11892-019-1270-y. PMID: 31776781. PMCID: PMC6881429.
Article5. Eto K, Ohya Y, Nakamura Y, Abe I, Fujishima M. 2001; Comparative actions of insulin sensitizers on ion channels in vascular smooth muscle. Eur J Pharmacol. 423:1–7. DOI: 10.1016/S0014-2999(01)01047-0. PMID: 11438300.
Article6. Knock GA, Mishra SK, Aaronson PI. 1999; Differential effects of insulin-sensitizers troglitazone and rosiglitazone on ion currents in rat vascular myocytes. Eur J Pharmacol. 368:103–109. DOI: 10.1016/S0014-2999(99)00020-5. PMID: 10096775.
Article7. Ahn HS, Kim SE, Jang HJ, Kim MJ, Rhie DJ, Yoon SH, Jo YH, Kim MS, Sung KW, Kim SY, Hahn SJ. 2007; Open channel block of Kv1.3 by rosiglitazone and troglitazone: Kv1.3 as the pharmacological target for rosiglitazone. Naunyn Schmiedebergs Arch Pharmacol. 374:305–309. DOI: 10.1007/s00210-006-0118-6. PMID: 17119927.
Article8. Jeong I, Choi BH, Hahn SJ. 2011; Rosiglitazone inhibits Kv4.3 potassium channels by open-channel block and acceleration of closed-state inactivation. Br J Pharmacol. 163:510–520. DOI: 10.1111/j.1476-5381.2011.01210.x. PMID: 21232039. PMCID: PMC3101614.
Article9. Overturf KE, Russell SN, Carl A, Vogalis F, Hart PJ, Hume JR, Sanders KM, Horowitz B. 1994; Cloning and characterization of a Kv1.5 delayed rectifier K+ channel from vascular and visceral smooth muscles. Am J Physiol. 267(5 Pt 1):C1231–C1238. DOI: 10.1152/ajpcell.1994.267.5.C1231. PMID: 7977686.10. Tamkun MM, Knoth KM, Walbridge JA, Kroemer H, Roden DM, Glover DM. 1991; Molecular cloning and characterization of two voltage-gated K+ channel cDNAs from human ventricle. FASEB J. 5:331–337. DOI: 10.1096/fasebj.5.3.2001794. PMID: 2001794.
Article11. Deal KK, England SK, Tamkun MM. 1996; Molecular physiology of cardiac potassium channels. Physiol Rev. 76:49–67. DOI: 10.1152/physrev.1996.76.1.49. PMID: 8592732.
Article12. Colatsky TJ, Follmer CH, Starmer CF. 1990; Channel specificity in antiarrhythmic drug action. Mechanism of potassium channel block and its role in suppressing and aggravating cardiac arrhythmias. Circulation. 82:2235–2242. DOI: 10.1161/01.CIR.82.6.2235. PMID: 2242545.
Article13. Li GR, Feng J, Wang Z, Fermini B, Nattel S. 1996; Adrenergic modulation of ultrarapid delayed rectifier K+ current in human atrial myocytes. Circ Res. 78:903–915. DOI: 10.1161/01.RES.78.5.903. PMID: 8620611.
Article14. Wang Z, Fermini B, Nattel S. 1993; Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 73:1061–1076. DOI: 10.1161/01.RES.73.6.1061. PMID: 8222078.
Article15. Cobbe SM. 1994; Incidence and risks associated with atrial fibrillation. Pacing Clin Electrophysiol. 17(5 Pt 2):1005–1010. DOI: 10.1111/j.1540-8159.1994.tb01453.x. PMID: 7518586.
Article16. Swanson R, Marshall J, Smith JS, Williams JB, Boyle MB, Folander K, Luneau CJ, Antanavage J, Oliva C, Buhrow SA, Bennet C, Stein RB, Kaczmarek LK. 1990; Cloning and expression of cDNA and genomic clones encoding three delayed rectifier potassium channels in rat brain. Neuron. 4:929–939. DOI: 10.1016/0896-6273(90)90146-7. PMID: 2361015.
Article17. Choi BH, Choi JS, Jeong SW, Hahn SJ, Yoon SH, Jo YH, Kim MS. 2000; Direct block by bisindolylmaleimide of rat Kv1.5 expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 293:634–640. PMID: 10773038.18. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. 1981; Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391:85–100. DOI: 10.1007/BF00656997. PMID: 6270629.
Article19. Snyders DJ, Yeola SW. 1995; Determinants of antiarrhythmic drug action. Electrostatic and hydrophobic components of block of the human cardiac hKv1.5 channel. Circ Res. 77:575–583. DOI: 10.1161/01.RES.77.3.575. PMID: 7641327.20. Delpón E, Valenzuela C, Gay P, Franqueza L, Snyders DJ, Tamargo J. 1997; Block of human cardiac Kv1.5 channels by loratadine: voltage-, time- and use-dependent block at concentrations above therapeutic levels. Cardiovasc Res. 35:341–350. DOI: 10.1016/S0008-6363(97)00121-1. PMID: 9349397.
Article21. Valenzuela C, Delpón E, Franqueza L, Gay P, Pérez O, Tamargo J, Snyders DJ. 1996; Class III antiarrhythmic effects of zatebradine. Time-, state-, use-, and voltage-dependent block of hKv1.5 channels. Circulation. 94:562–570. DOI: 10.1161/01.CIR.94.3.562. PMID: 8759103.22. Franqueza L, Valenzuela C, Delpón E, Longobardo M, Caballero R, Tamargo J. 1998; Effects of propafenone and 5-hydroxy-propafenone on hKv1.5 channels. Br J Pharmacol. 125:969–978. DOI: 10.1038/sj.bjp.0702129. PMID: 9846634. PMCID: PMC1565661.
Article23. Park J, Cho KH, Lee HJ, Choi JS, Rhie DJ. 2020; Open channel block of Kv1.4 potassium channels by aripiprazole. Korean J Physiol Pharmacol. 24:545–553. DOI: 10.4196/kjpp.2020.24.6.545. PMID: 33093275. PMCID: PMC7585592.
Article24. Lee HM, Hahn SJ, Choi BH. 2016; Blockade of Kv1.5 by paroxetine, an antidepressant drug. Korean J Physiol Pharmacol. 20:75–82. DOI: 10.4196/kjpp.2016.20.1.75. PMID: 26807026. PMCID: PMC4722194.
Article25. Lee HM, Hahn SJ, Choi BH. 2016; Blockade of Kv1.5 channels by the antidepressant drug sertraline. Korean J Physiol Pharmacol. 20:193–200. DOI: 10.4196/kjpp.2016.20.2.193. PMID: 26937216. PMCID: PMC4770110.
Article26. Kolte BL, Raut BB, Deo AA, Bagool MA, Shinde DB. 2003; Liquid chromatographic method for the determination of rosiglitazone in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 788:37–44. DOI: 10.1016/S1570-0232(02)01011-5. PMID: 12668069.
Article27. Miles PD, Barak Y, Evans RM, Olefsky JM. 2003; Effect of heterozygous PPARgamma deficiency and TZD treatment on insulin resistance associated with age and high-fat feeding. Am J Physiol Endocrinol Metab. 284:E618–E626. DOI: 10.1152/ajpendo.00312.2002. PMID: 12556354.28. Tontonoz P, Spiegelman BM. 2008; Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 77:289–312. DOI: 10.1146/annurev.biochem.77.061307.091829. PMID: 18518822.29. Lebovitz HE, Banerji MA. 2001; Insulin resistance and its treatment by thiazolidinediones. Recent Prog Horm Res. 56:265–294. DOI: 10.1210/rp.56.1.265. PMID: 11237217.
Article30. Fedida D, Eldstrom J, Hesketh JC, Lamorgese M, Castel L, Steele DF, Van Wagoner DR. 2003; Kv1.5 is an important component of repolarizing K+ current in canine atrial myocytes. Circ Res. 93:744–751. DOI: 10.1161/01.RES.0000096362.60730.AE. PMID: 14500335.
Article31. Chapelsky MC, Thompson-Culkin K, Miller AK, Sack M, Blum R, Freed MI. 2003; Pharmacokinetics of rosiglitazone in patients with varying degrees of renal insufficiency. J Clin Pharmacol. 43:252–259. DOI: 10.1177/0091270002250602. PMID: 12638393.
Article32. Hruska MW, Frye RF. 2004; Simplified method for determination of rosiglitazone in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 803:317–320. DOI: 10.1016/j.jchromb.2004.01.010. PMID: 15063342.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of rosiglitazone, an antidiabetic drug, on Kv3.1 channels
- Effects of 3,3′,4,4′,5-pentachlorobiphenyl on human Kv1.3 and Kv1.5 channels
- Regulation of Antiarrhythmic Drug Propafenone Effects on the C-type KV1.4 Potassium Channel by PHo and K+
- Open channel block of Kv1.4 potassium channels by aripiprazole
- Encainide, a class Ic anti-arrhythmic agent, blocks voltage-dependent potassium channels in coronary artery smooth muscle cells