Korean J Physiol Pharmacol.
2012 Oct;16(5):327-332. 10.4196/kjpp.2012.16.5.327.
Wide Spectrum of Inhibitory Effects of Sertraline on Cardiac Ion Channels
- Affiliations
-
- 1Next-Generation Pharmaceutical Research Center, Korea Institute of Toxicology, Korea Research Institute of Chemical Technology, Daejeon 305-600, Korea.
- 2Department of Physiology and Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul 110-799, Korea. sjoonkim@snu.ac.kr
- KMID: 1493965
- DOI: http://doi.org/10.4196/kjpp.2012.16.5.327
Abstract
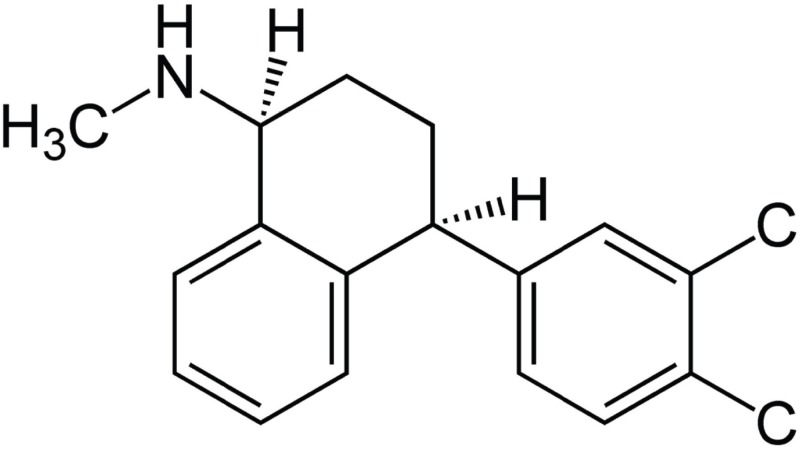
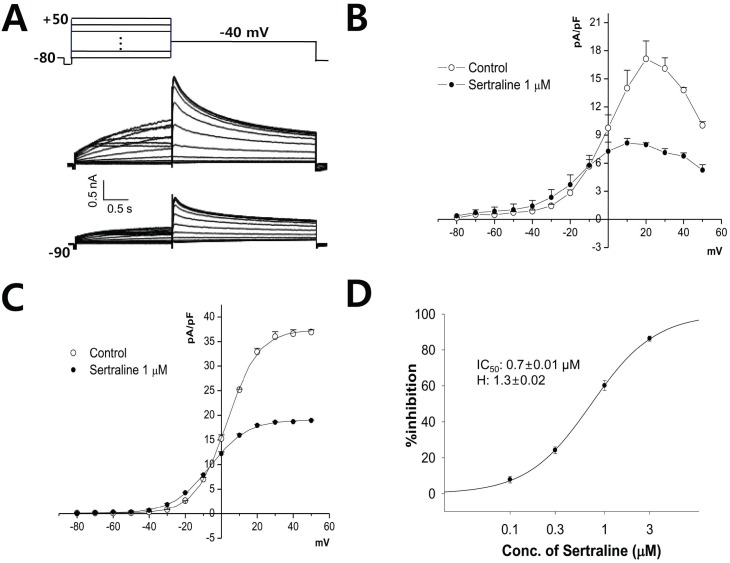
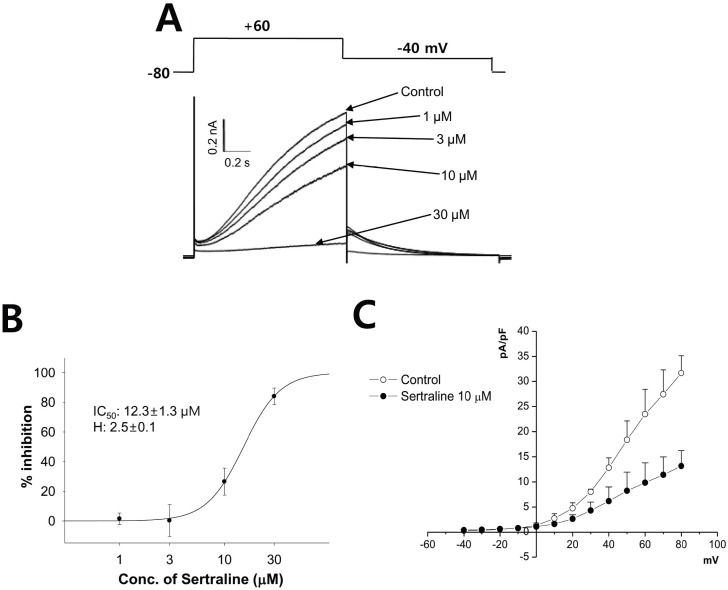
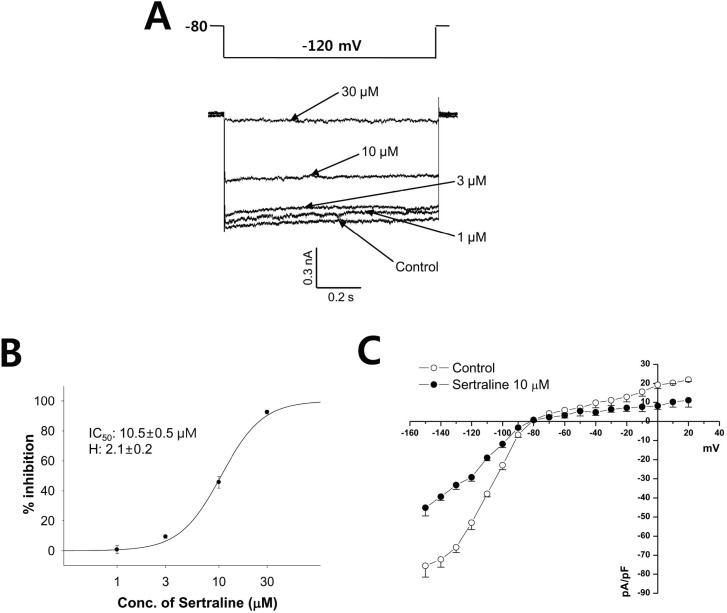
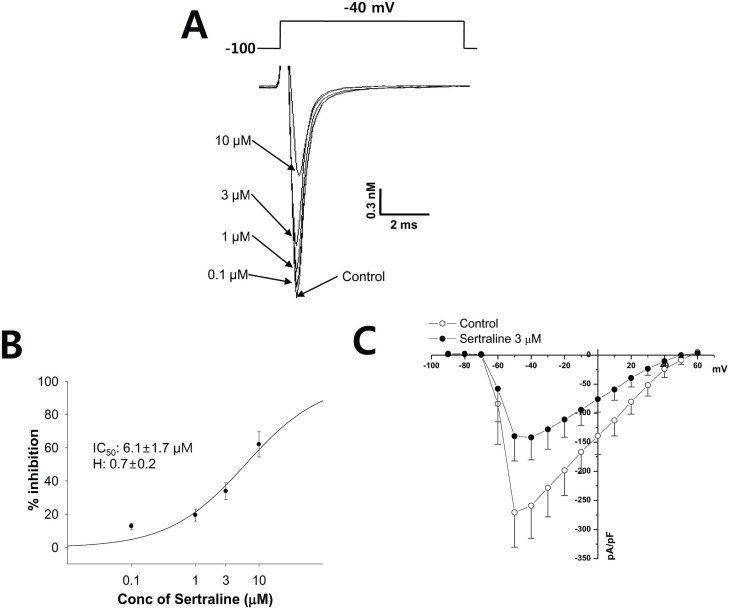
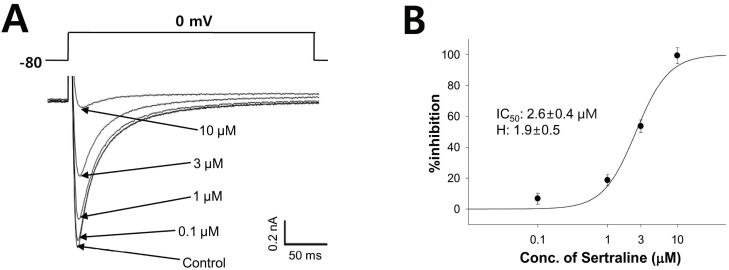
- Sertraline is a commonly used antidepressant of the selective serotonin reuptake inhibitors (SSRIs) class. In these experiments, we have used the whole cell patch clamp technique to examine the effects of sertraline on the major cardiac ion channels expressed in HEK293 cells and the native voltage-gated Ca2+ channels in rat ventricular myocytes. According to the results, sertraline is a potent blocker of cardiac K+ channels, such as hERG, IKs and IK1. The rank order of inhibitory potency was hERG >IK1> IKs with IC50 values of 0.7, 10.5, and 15.2 microM, respectively. In addition to K+ channels, sertraline also inhibited INa and ICa, and the IC50 values are 6.1 and 2.6 microM, respectively. Modification of these ion channels by sertraline could induce changes of the cardiac action potential duration and QT interval, and might result in cardiac arrhythmia.
MeSH Terms
Figure
Cited by 2 articles
-
Taxifolin Glycoside Blocks Human
ether-a-go-go Related Gene K+ Channels
Jihyun Yun, Hyemi Bae, Sun Eun Choi, Jung-Ha Kim, Young Wook Choi, Inja Lim, Chung Soo Lee, Min Won Lee, Jae-Hong Ko, Seong Jun Seo, Hyoweon Bang
Korean J Physiol Pharmacol. 2013;17(1):37-42. doi: 10.4196/kjpp.2013.17.1.37.Blockade of Kv1.5 channels by the antidepressant drug sertraline
Hyang Mi Lee, Sang June Hahn, Bok Hee Choi
Korean J Physiol Pharmacol. 2016;20(2):193-200. doi: 10.4196/kjpp.2016.20.2.193.
Reference
-
1. Viskin S. Long QT syndromes and torsade de pointes. Lancet. 1999; 354:1625–1633. PMID: 10560690.
Article2. Kannankeril PJ, Roden DM. Drug-induced long QT and torsade de pointes: recent advances. Curr Opin Cardiol. 2007; 22:39–43. PMID: 17143043.
Article3. Witchel HJ, Hancox JC, Nutt DJ. Psychotropic drugs, cardiac arrhythmia, and sudden death. J Clin Psychopharmacol. 2003; 23:58–77. PMID: 12544377.
Article4. Glassman AH, Bigger JT Jr. Cardiovascular effects of therapeutic doses of tricyclic antidepressants. A review. Arch Gen Psychiatry. 1981; 38:815–820. PMID: 7247643.5. Yeragani VK, Roose S, Mallavarapu M, Radhakrishna RK, Pesce V. Major depression with ischemic heart disease: effects of paroxetine and nortriptyline on measures of nonlinearity and chaos of heart rate. Neuropsychobiology. 2002; 46:125–135. PMID: 12422059.
Article6. Yeragani VK. Major depression and long-term heart period variability. Depress Anxiety. 2000; 12:51–52. PMID: 10999246.
Article7. Rodriguez de la Torre B, Dreher J, Malevany I, Bagli M, Kolbinger M, Omran H, Lüderitz B, Rao ML. Serum levels and cardiovascular effects of tricyclic antidepressants and selective serotonin reuptake inhibitors in depressed patients. Ther Drug Monit. 2001; 23:435–440. PMID: 11477329.8. Park KS, Kong ID, Chung HS, Joo NY, Park KC, Lee JW. Effects of antidepressants on cardiac functions in the perfused rat heart. J Wonju Coll Med. 1996; 9:176–181.9. Park KS, Kong ID, Park KC, Lee JW. Fluoxetine inhibits L-type Ca2+ and transient outward K+ currents in rat ventricular myocytes. Yonsei Med J. 1999; 40:144–151. PMID: 10333718.10. Fisch C, Knoebel SB. Electrocardiographic findings in sertraline depression trials. Drug Invest. 1992; 4:305–312.
Article11. Amin M, Lehmann H, Mirmiran J. A double-blind, placebo-controlled dose-finding study with sertraline. Psychopharmacol Bull. 1989; 25:164–167. PMID: 2690162.12. Hoehns JD, Fouts MM, Kelly MW, Tu KB. Sudden cardiac death with clozapine and sertraline combination. Ann Pharmacother. 2001; 35:862–866. PMID: 11485134.
Article13. Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001; 104:569–580. PMID: 11239413.
Article14. Tseng GN. I(Kr): the hERG channel. J Mol Cell Cardiol. 2001; 33:835–849. PMID: 11343409.
Article15. Saldeen J, Curiel DT, Eizirik DL, Andersson A, Strandell E, Buschard K, Welsh N. Efficient gene transfer to dispersed human pancreatic islet cells in vitro using adenovirus-polylysine/DNA complexes or polycationic liposomes. Diabetes. 1996; 45:1197–1203. PMID: 8772722.16. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981; 391:85–100. PMID: 6270629.
Article17. Meijer WE, Heerdink ER, Leufkens HG, Herings RM, Egberts AC, Nolen WA. Incidence and determinants of long-term use of antidepressants. Eur J Clin Pharmacol. 2004; 60:57–61. PMID: 14985889.
Article18. Kelsey JE, Nemeroff CB. Sadock BJ, Sadock VA, editors. Selective serotonin reuptake inhibitors: introduction and overview. 2000. . Philadelphia PA: Lippincott Williams & Willis;p. 2432–2435.19. Maertens C, Droogmans G, Verbesselt R, Nilius B. Block of volume-regulated anion channels by selective serotonin reuptake inhibitors. Naunyn Schmiedebergs Arch Pharmacol. 2002; 366:158–165. PMID: 12122503.20. Wang GK, Mitchell J, Wang SY. Block of persistent late Na+ currents by antidepressant sertraline and paroxetine. J Membr Biol. 2008; 222:79–90. PMID: 18418539.21. Kobayashi T, Washiyama K, Ikeda K. Inhibition of G protein-activated inwardly rectifying K+ channels by different classes of antidepressants. PLoS One. 2011; 6:e28208. PMID: 22164246.22. Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995; 81:299–307. PMID: 7736582.24. Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, Siegl PK, Strang I, Sullivan AT, Wallis R, Camm AJ, Hammond TG. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003; 58:32–45. PMID: 12667944.
Article25. Chen J, Zheng R, Melman YF, McDonald TV. Functional interactions between KCNE1 C-terminus and the KCNQ1 channel. PLoS One. 2009; 4:e5143. PMID: 19340287.
Article26. Li GR, Lau CP, Ducharme A, Tardif JC, Nattel S. Transmural action potential and ionic current remodeling in ventricles of failingcanine hearts. Am J Physiol Heart Circ Physiol. 2002; 283:H1031–H1041. PMID: 12181133.27. Jiang M, Zhang M, Tang DG, Clemo HF, Liu J, Holwitt D, Kasirajan V, Pond AL, Wettwer E, Tseng GN. KCNE2 protein is expressed in ventricles of different species, and changes in its expression contribute to electrical remodeling in diseased hearts. Circulation. 2004; 109:1783–1788. PMID: 15066947.
Article28. Näbauer M, Kääb S. Potassium channel down-regulation in heart failure. Cardiovasc Res. 1998; 37:324–334. PMID: 9614489.
Article29. Tsuboi M, Antzelevitch C. Cellular basis for electrocardiographic and arrhythmic manifestations of Andersen-Tawil syndrome (LQT7). Heart Rhythm. 2006; 3:328–335. PMID: 16500306.
Article30. Grant AO, Carboni MP, Neplioueva V, Starmer CF, Memmi M, Napolitano C, Priori S. Long QT syndrome, Brugada syndrome, and conduction system disease are linked to a single sodium channel mutation. J Clin Invest. 2002; 110:1201–1209. PMID: 12393856.
Article31. Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, Sanguinetti MC, Keating MT. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci USA. 2005; 102:8089–8096. PMID: 15863612.
Article32. Pacher P, Ungvari Z, Nanasi PP, Furst S, Kecskemeti V. Speculations on difference between tricyclic and selective serotonin reuptake inhibitor antidepressants on their cardiac effects. Is there any? Curr Med Chem. 1999; 6:469–480. PMID: 10213794.33. De Ponti F, Poluzzi E, Cavalli A, Recanatini M, Montanaro N. Safety of non-antiarrhythmic drugs that prolong the QT interval or induce torsade de pointes: an overview. Drug Saf. 2002; 25:263–286. PMID: 11994029.34. Haddad PM, Anderson IM. Antipsychotic-related QTc prolongation, torsade de pointes and sudden death. Drugs. 2002; 62:1649–1671. PMID: 12109926.
Article35. Witchel HJ, Hancox JC, Nutt DJ. Psychotropic drugs, cardiac arrhythmia, and sudden death. J Clin Psychopharmacol. 2003; 23:58–77. PMID: 12544377.
Article36. Pousti A, Bakhtiarian A, Najafi R, Deemyad T, Brumand K, Hosseini MJ. Effect of sertraline on ouabain-induced arrhythmia in isolated guinea-pig atria. Depress Anxiety. 2009; 26:E106–E110. PMID: 19242981.
Article37. Amin M, Lehmann H, Mirmiran J. A double-blind, placebo-controlled dose-finding study with sertraline. Psychopharmacol Bull. 1989; 25:164–167. PMID: 2690162.38. de Boer RA, van Dijk TH, Holman ND, van Melle JP. QT interval prolongation after sertraline overdose: a case report. BMC Emerg Med. 2005; 5:5. PMID: 16029494.
Article39. Kocabay G, Yildiz M, Eksi Duran N, Ozkan M. Sinus arrest due to sertraline. Clin Cardiol. 2010; 33:E114–E115. PMID: 20552630.
Article40. Patel NH, Golwala H, Stavrakis S, Schechter E. Sertraline-Induced Ventricular Tachycardia. Am J Ther. 2011; [Epub ahead of print].
Article